
Quality control
Conducting experimental research and quality assurance/quality control studies on clinical positron emission tomography/computed tomography (PET/CT) and PET/magnetic resonance (MR) scanners

Multi-parametric imaging for enhanced diagnosis and treatment response monitoring
Developing novel and clinically adoptable whole-body dynamic positron emission tomography (PET) imaging protocols for total-body multi-parametric PET imaging using widely accessible limited axial field of view clinical PET scanners of today

Research: optimal reconstruction settings and artificial intelligence-driven tools
Goal: To recover positron emission tomography (PET) image quality in data acquired via long axial field-of-view PET scanners cost-effectively
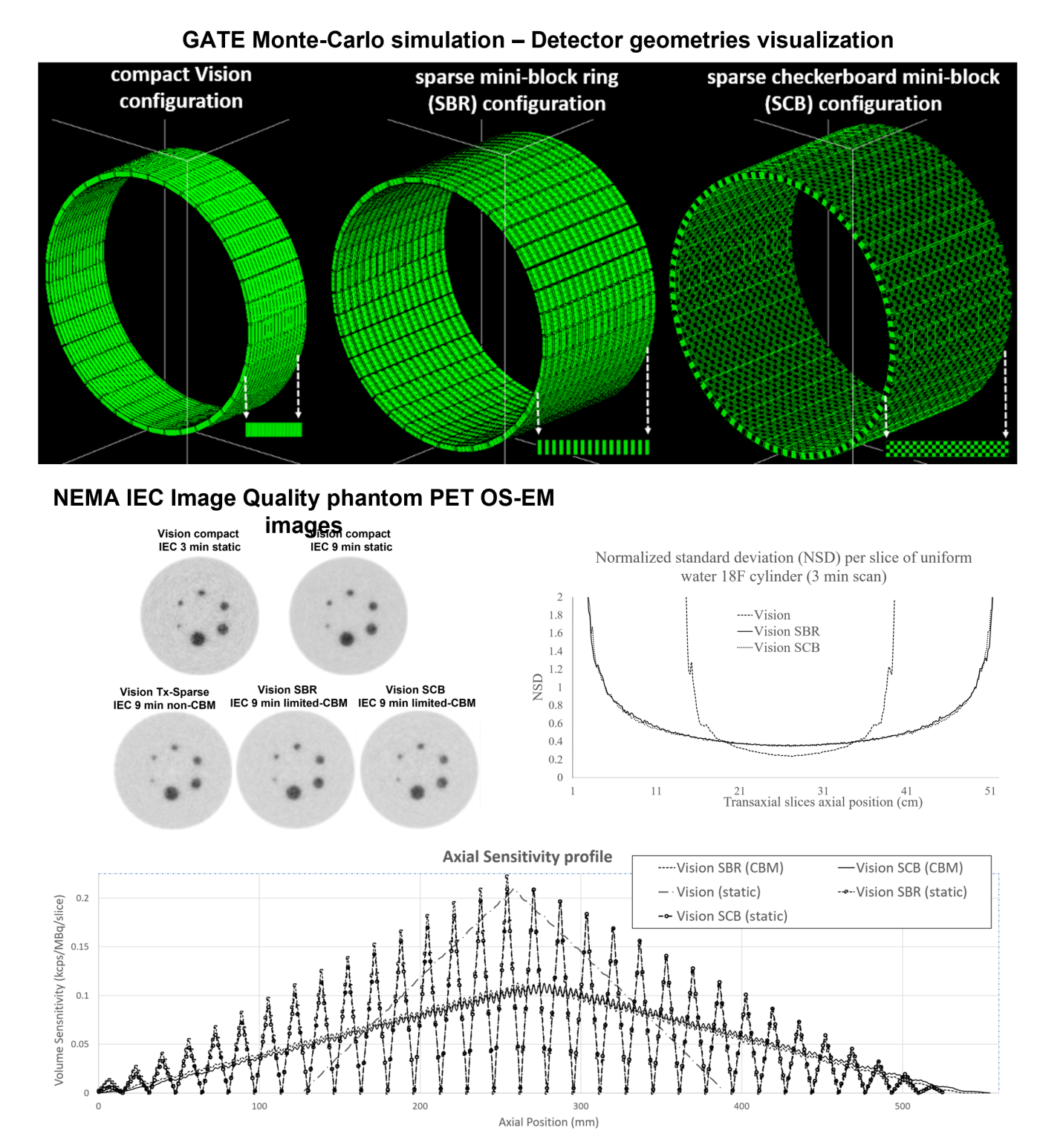
Adaptively sparse PET scanner geometry
Designing computationally efficient Monte Carlo simulations to evaluate physical performance of cost-effective axial field of view positron emission tomography (PET) scanners

Quantifying human arterial input function
Validating performance of SynchroPET™ arterial positron emission tomography (PET) ring scanner in quantifying human arterial input function quantification by non-invasively imaging the human wrist outside a PET scanner.

Radioactive phantom experiments
Designing radioactive phantom experiments to validate novel positron emission tomography (PET) scan protocols, quantitative image reconstruction, and data analysis methods


