Yi Wang, Ph.D., is the Director of the Magnetic Resonance Imaging Research Institute (MRIRI). He has invented multiple MRI technologies of great importance to the clinical and scientific communities, including, a) quantitative susceptibility mapping (QSM), which has led to a new MRI discipline for studying tissue magnetism, b) the stepping table platform with multiple local coils for large field of view (FOV) imaging, and c) quantitative transport mapping (QTM).
Lab Focus
Major research interests in Professor Wang’s lab involve applying and developing data science, machine learning, optimization, physics, and statistical inference techniques for medical imaging acquisition and analysis. These include increasing imaging speed, reducing image artifacts, and generating novel image contrasts/biomarkers using computer vision and signal processing strategies. The lab seeks to formulate medical imaging problems for disease diagnosis and therapy delivery, as inverse problems from acquired signals to underlying pathogeneses based on biophysics. The lab works closely with clinicians to study neurological diseases such as multiple sclerosis (MS), Parkinson’s disease (PD), Alzheimer’s disease (AD), stroke, cancer in various organs, and liver and heart diseases. The inverse problems are often poorly conditioned and involve noisy incomplete data, resulting in reconstructed images with errors or artifacts commonly encountered in computer vision. The lab has developed the Bayesian statistical inference approach to removing image artifacts in MRI using prior knowledge established in biomedicine, or acquired from multiple imaging modalities, including immunohistochemical staining and optical imaging.
The lab’s work is exemplified by the following:
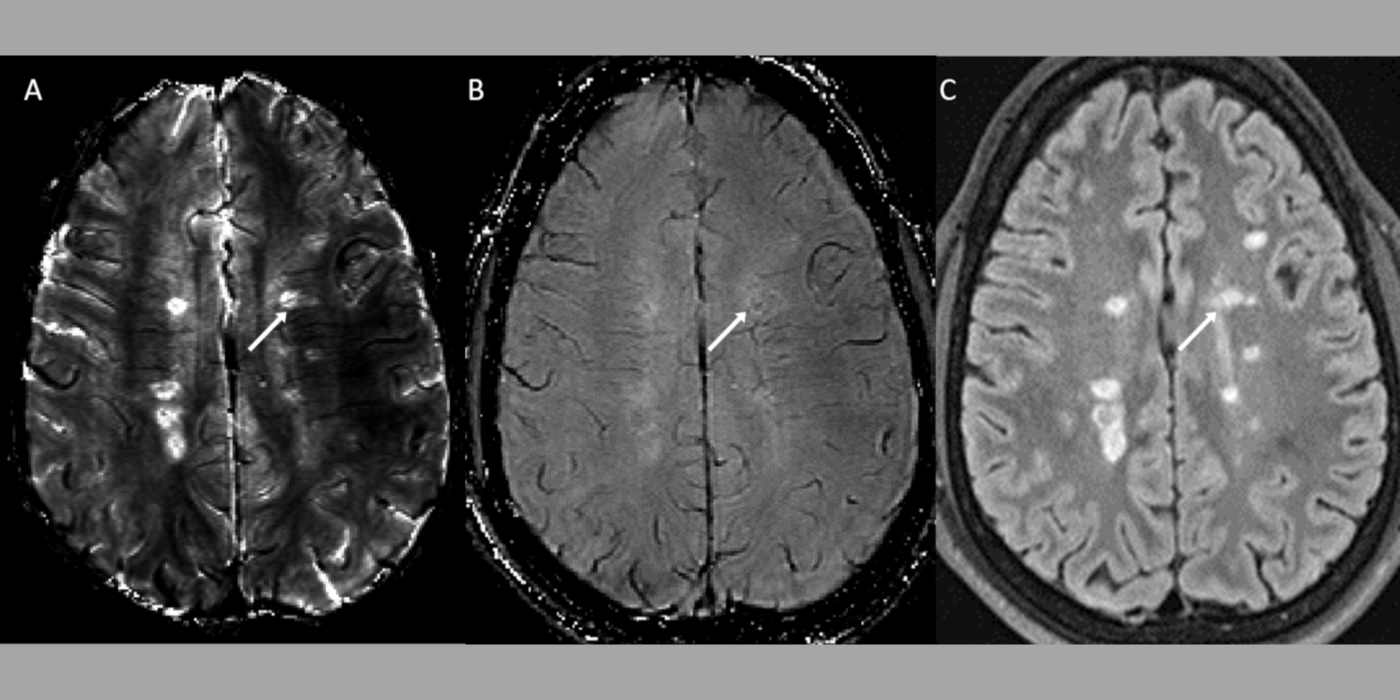
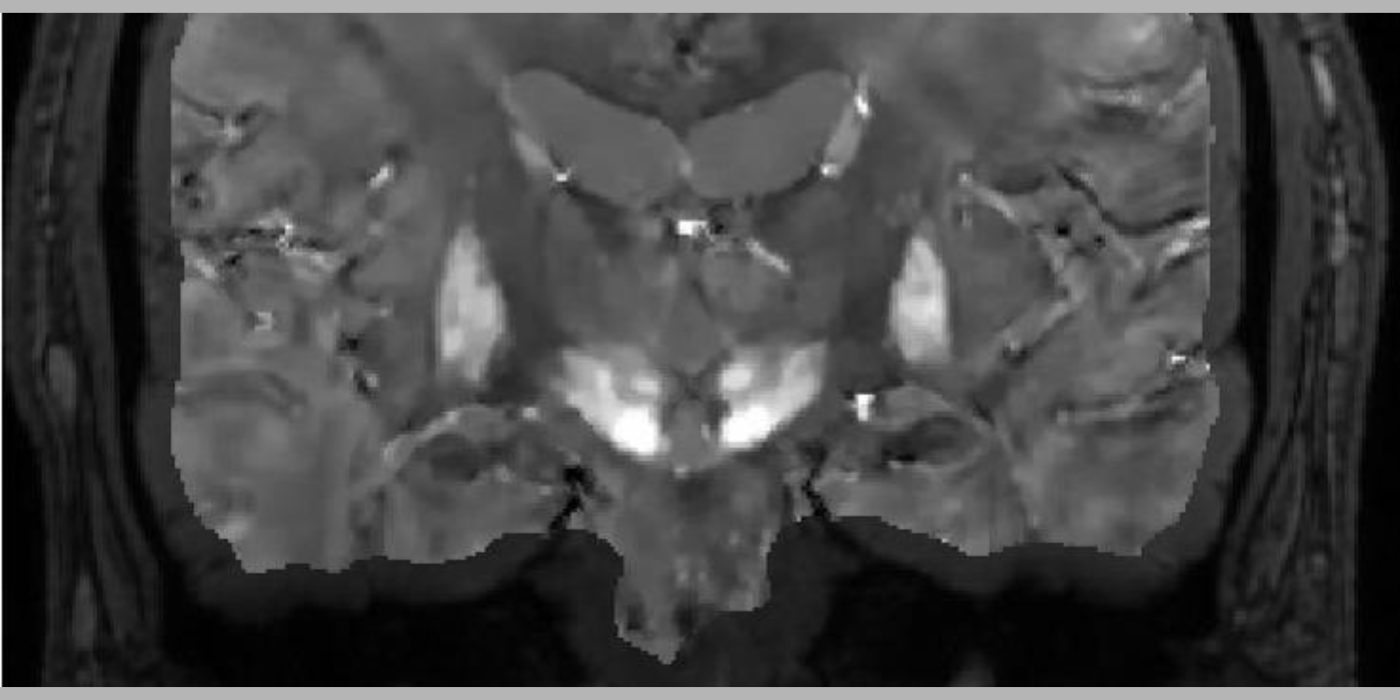
QSM to solve the field-to-susceptibility inverse problem using the Bayesian approach. Tissue susceptibility reflects molecular electron cloud properties, and QSM enables its precise quantitative study. QSM has become a very active field of studying tissue magnetism for applications in neurodegeneration, inflammation, oxygen consumption, hemorrhage, liver, osteoporosis, atherosclerosis and drug delivery. QSM is increasingly used in clinical practice, particularly in precision targeting for deep brain stimulation, precision monitoring of chronic active hemorrhages and inflammation, precision medication for iron chelation therapy, and precision diagnosis and gadolinium-free imaging for multiple sclerosis.
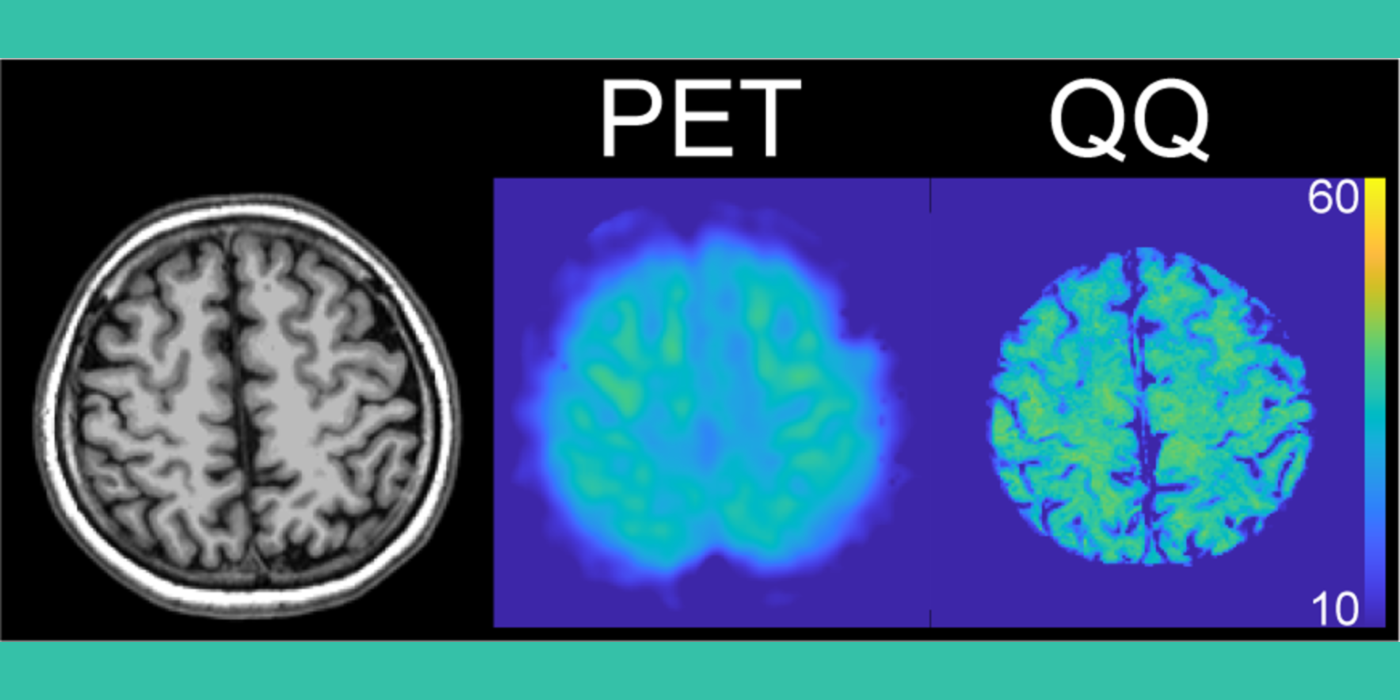
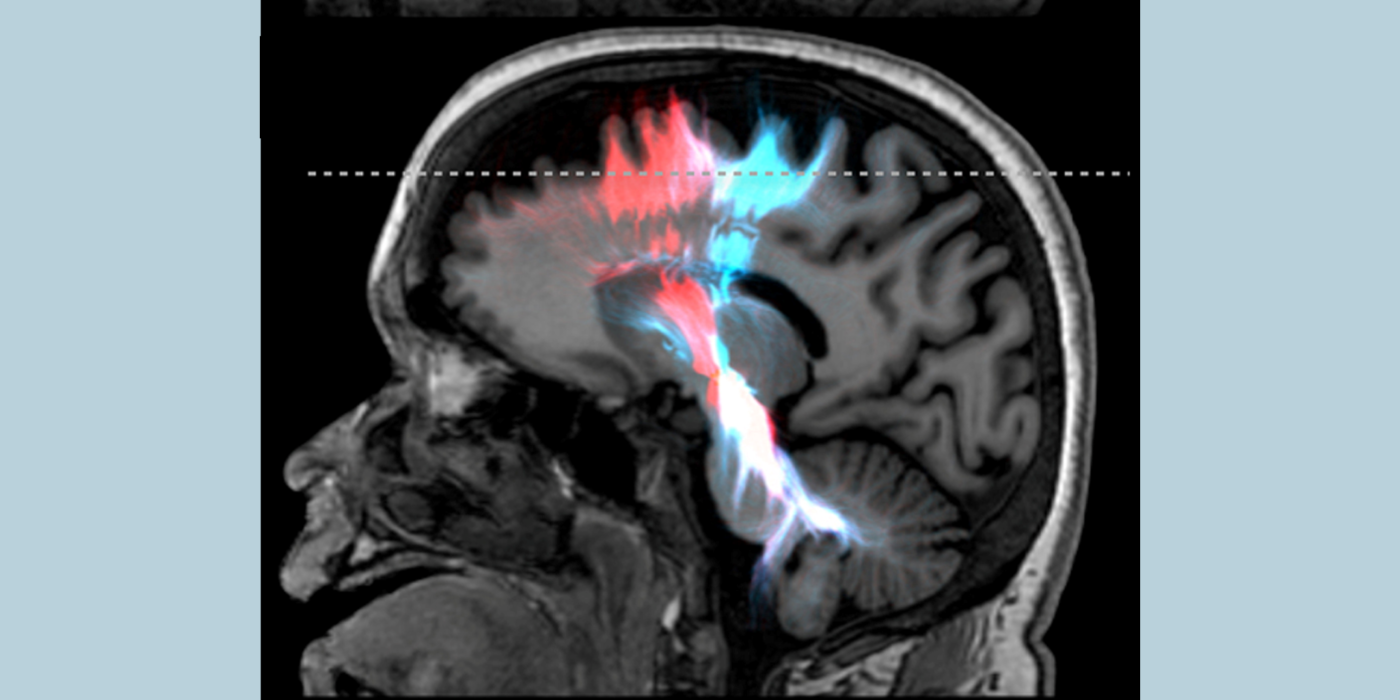
QTM to solve the inverse problem from imaging to tissue perfusion quantification. The lab develops fast dynamic imaging (4D) to capture tracer (drugs, contrast agents, and spin labels) transport in tissue using super resolution, sparsity, and deep learning techniques. Perfusion parameters affect imaging through convolution in space time according to transport equation of mass and momentum flux laws. The lab develops QTM to deconvolve 4D time-resolved imaging for perfusion quantification. QTM enables precise measurement of blood flow in tissue and helps with precise delivery of therapeutic drugs, cryotherapy, and tissue ablation.
Lesion segmentation from acquired images to enable automated precise measurements and analyses of disease burden. The lab employs various image segmentation techniques including image feature-based approaches and deep neural network-based approaches.
Collaborators
Gary Brittenham, M.D.
Susan Gauthier, D.O.
Manu Goyal, M.D.
Ajay Gupta, M.D.
Robert J. Min, M.D., M.B.A.
Martin Prince, M.D., Ph.D.
Sujit Sheth, M.D.
Alexander Shtilbans, M.D., Ph.D.
Yi Su, Ph.D.
Alumni
Priya Balasubramanian
Ryan Brown
Junghun Cho
Noel Codella
Mitchell Cooper
Deqi Cui
Kofi Deh
Jianwu Dong
Sarah Eskreis-Winkler
Yihao Guo
Ramin Jafari
Junhwan Kim
Gaiying Li
Jiahao Li
Jianqi Li
Tian Liu
Zhe Liu
Keigo Kawaji
Bryan Kressler
Mengchao Pei
Ashish Raj
Gurmeet Singh
Bo Xu
Lijia Wang
Yan Wen
Cynthia Wisnieff
Richard Wong
Jingwei Zhang
Zhenghui Zhang
Henry He Zhu
Yanchun Zhu
Code
- [LINK] to code for Zhang, J., et al., Temporal Feature Fusion with Sampling Pattern Optimization for Multi-echo Gradient Echo Acquisition and Image Reconstruction, International Conference on Medical Image Computing and Computer-Assisted Intervention, Springer, Cham: 2021.
- [LINK] to code for Zhang, J., et al., Bayesian learning of probabilistic dipole inversion for quantitative susceptibility mapping, Medical Imaging with Deep Learning, PMLR: 2020, and Probabilistic dipole inversion for adaptive quantitative susceptibility mapping, MELBA, MIDL: 2020 special issue, 2021.
- [LINK] to code for Zhang, J., et al., Hybrid optimization between iterative and network fine-tuning reconstructions for fast quantitative susceptibility mapping, Medical Imaging with Deep Learning, PMLR: 2021.
- [LINK] to code for Zhang, J., et al., Fidelity imposed network edit (FINE) for solving ill-posed image reconstruction, Neuroimage 211: (2020) 116579.
- [LINK] to code for Jafari, R., et al., Vastly accelerated linear least-squares fitting with numerical optimization for dual-input delay-compensated quantitative liver perfusion mapping, Magn. Reson Med.: 2018 Apr;79(4):2415-2421.
- [LINK] to code for Jafari, R., et al., Deep neural network for water/fat separation: Supervised training, unsupervised training, and no training, Magn. Reson. Med.: 2021 Apr;85(4):2263-2277.
- [LINK] to code for Zhang, J. et al., QSMRim-Net: Imbalance-aware learning for identification of chronic active multiple sclerosis lesions on quantitative susceptibility maps, NeuroImage: Clinical: 34 (2022) 102979.
- [LINK] to code for Zhang, j., et al., Rsanet: Recurrent slice-wise attention network for multiple sclerosis lesion segmentation, International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Cham: 2019.
- [LINK] to more Wang Lab code.