Research and Press Releases
Pretargeting: a path forward for radioimmunotherapy (in collaboration with Nai-Kong Cheung MD PhD of Memorial Sloan Kettering Cancer Center)
Curative therapy is a major need in solid human tumors of adults and children. Conventional radioimmunotherapy with tumor-targeting antibodies conjugated with radionuclides suffers from poor therapeutic index (TI). We use a multi-step approach to RIT called “pretargeted RIT” to improve TI by combining the desirable tumor-targeting features of antibodies with the ideal pharmacokinetics of small-molecule radiohaptens.
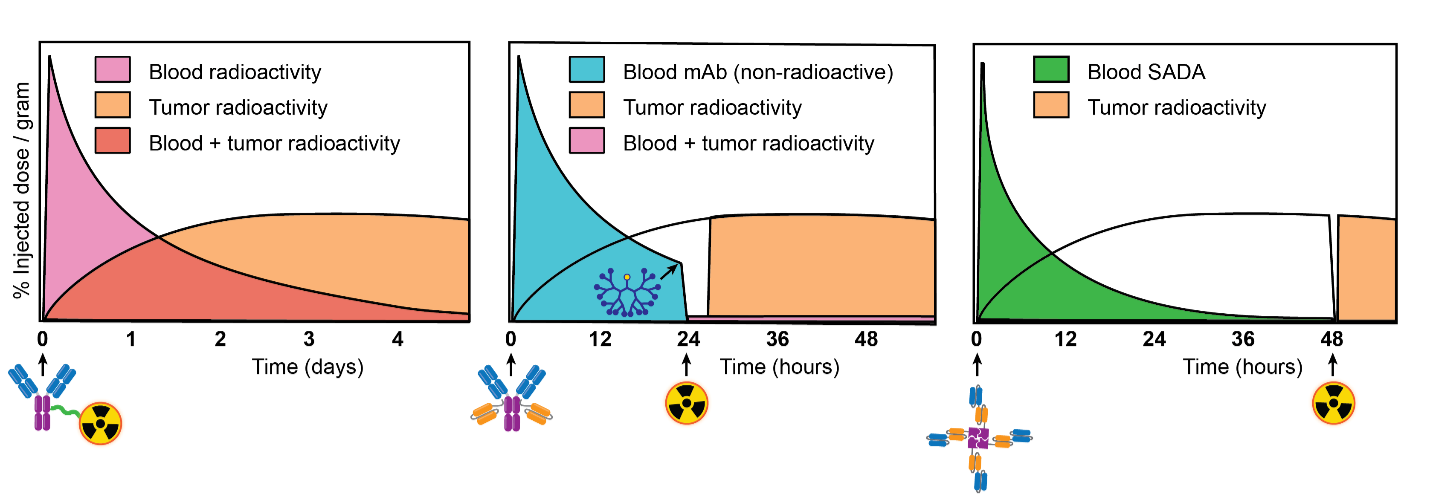
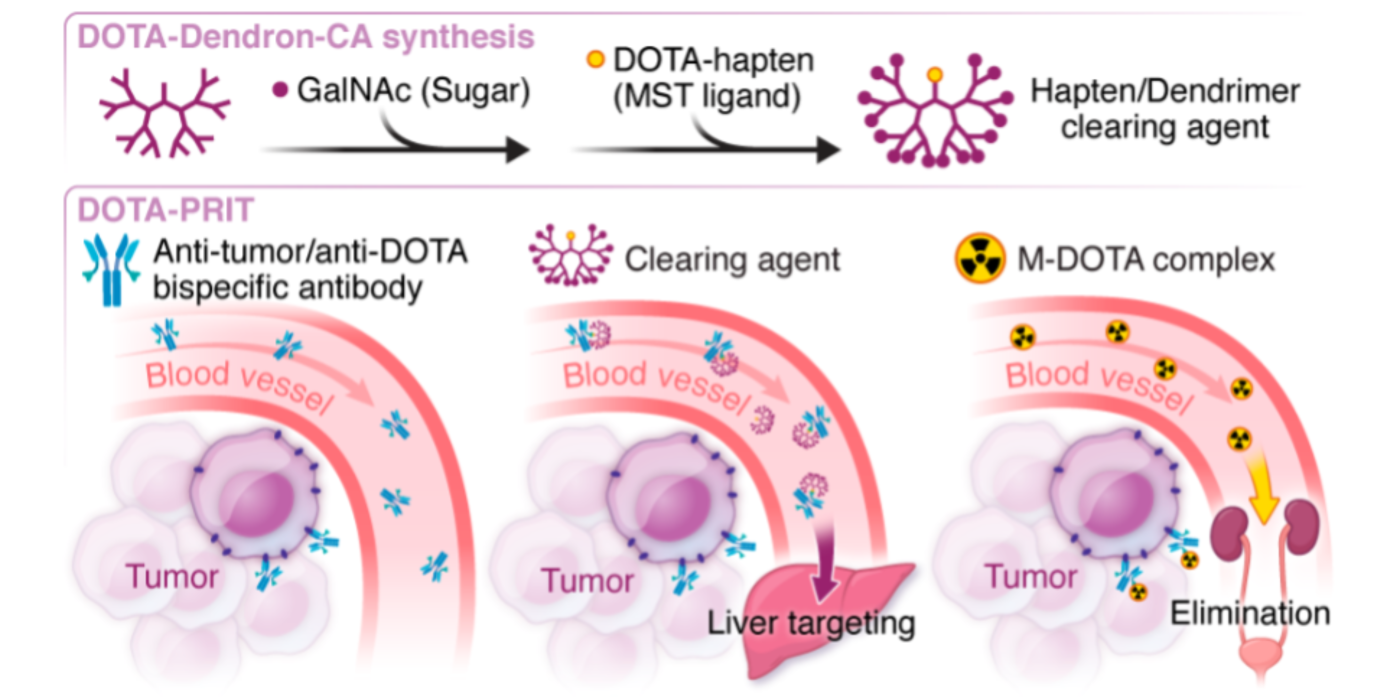
Fig 1. from PMID: 36215514 Conventional RIT (left) versus 3-step pretargeted RIT (center) versus 2-step pretargeted RIT (right). During conventional RIT, a radioimmunoconjugate is administered. Radioactive payload is specifically targeted to tumor, but at the expense of concurrent off-target exposure to normal tissues. During 3-step pretargeted RIT, a non-radioactive anti-tumor antigen/anti-radiohapten bispecific antibody (BsAb) is administered, followed the next day with a clearing agent to rapidly reduce circulating (i.e., not tumor bound) BsAb. After a few additional hours, a radiohapten is administered which is captured by intratumoral BsAb or otherwise efficiently cleared via the renal route. This dramatically reduces off-target exposure of radioactive payload. During 2-step pretargeted RIT, an innovative BsAb platform called “SADA” (self-assembling and disassembling) can be applied to eliminate the requirement of a clearing agent. PMID: 32958698
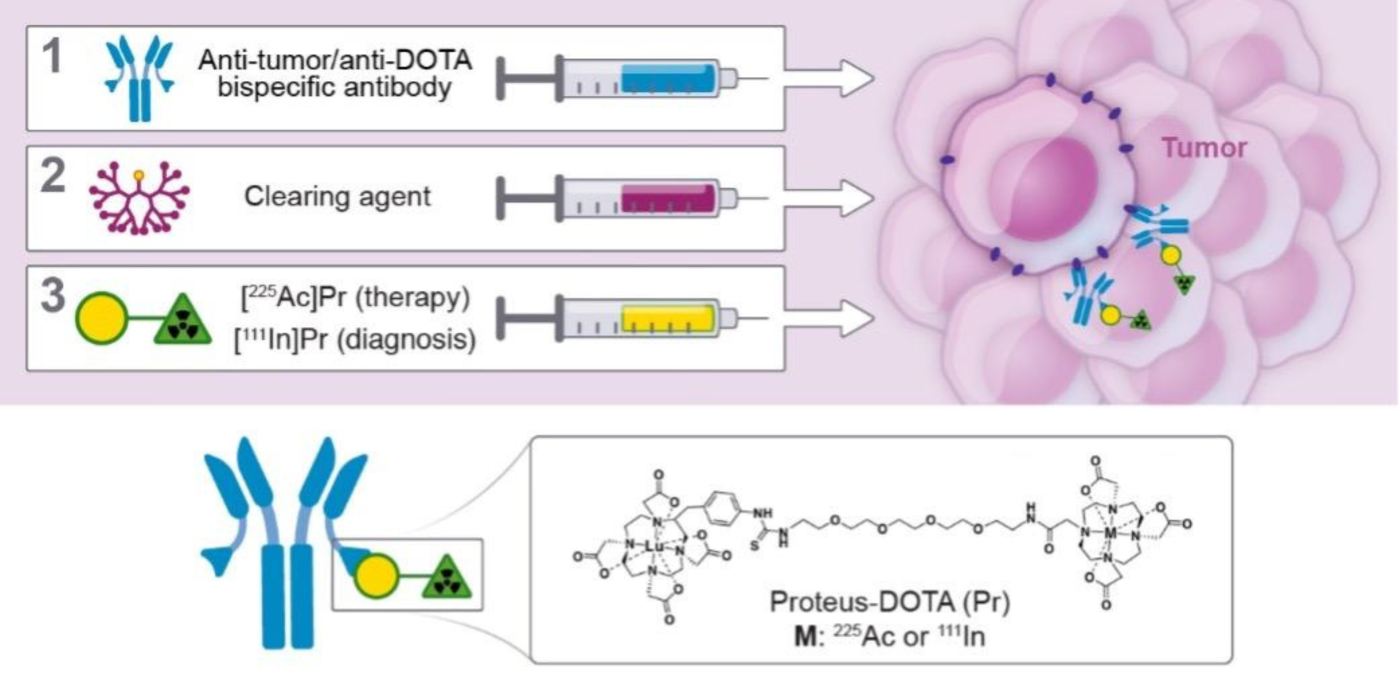
We strive to develop theranostic (therapy/diagnostics) PRIT with beta- or alpha-emitting radioisotopes to enable personalized dosimetry and treatment planning. In an approach we call “DOTA-PRIT” short for “1,4,7,10-tetraazacyclododecane-N, N’, N’’, N’’’-tetraacetic acid (DOTA)-based PRIT” we first administer a non-radioactive anti-tumor/anti-DOTA hapten bispecific antibodies to “pretarget” the tumor, followed by administration of a radioactive-DOTA-hapten.
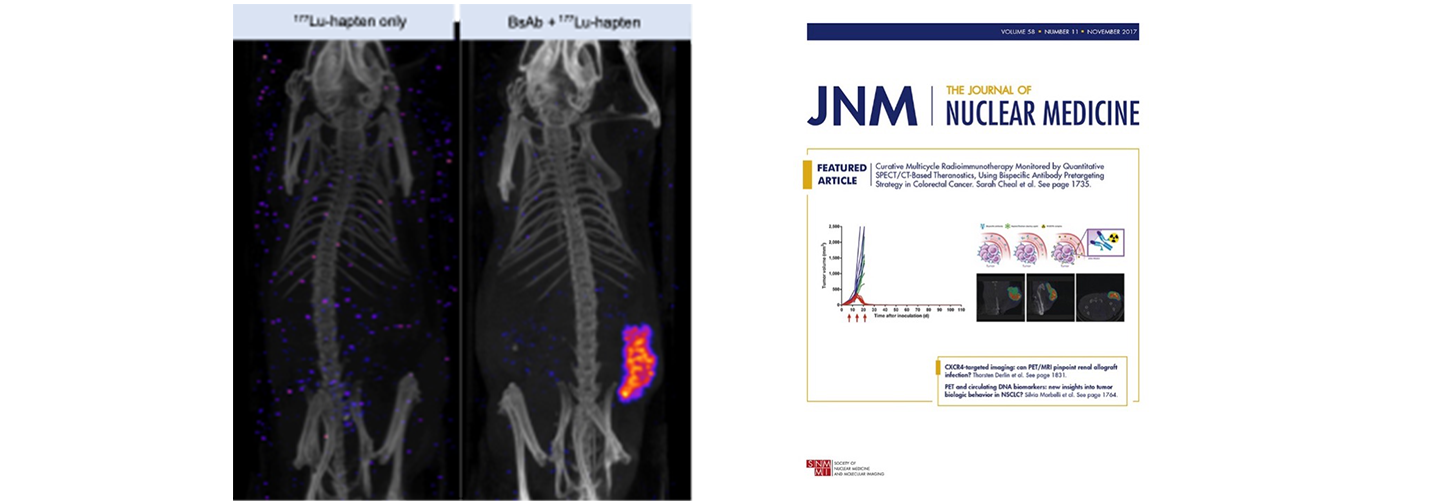
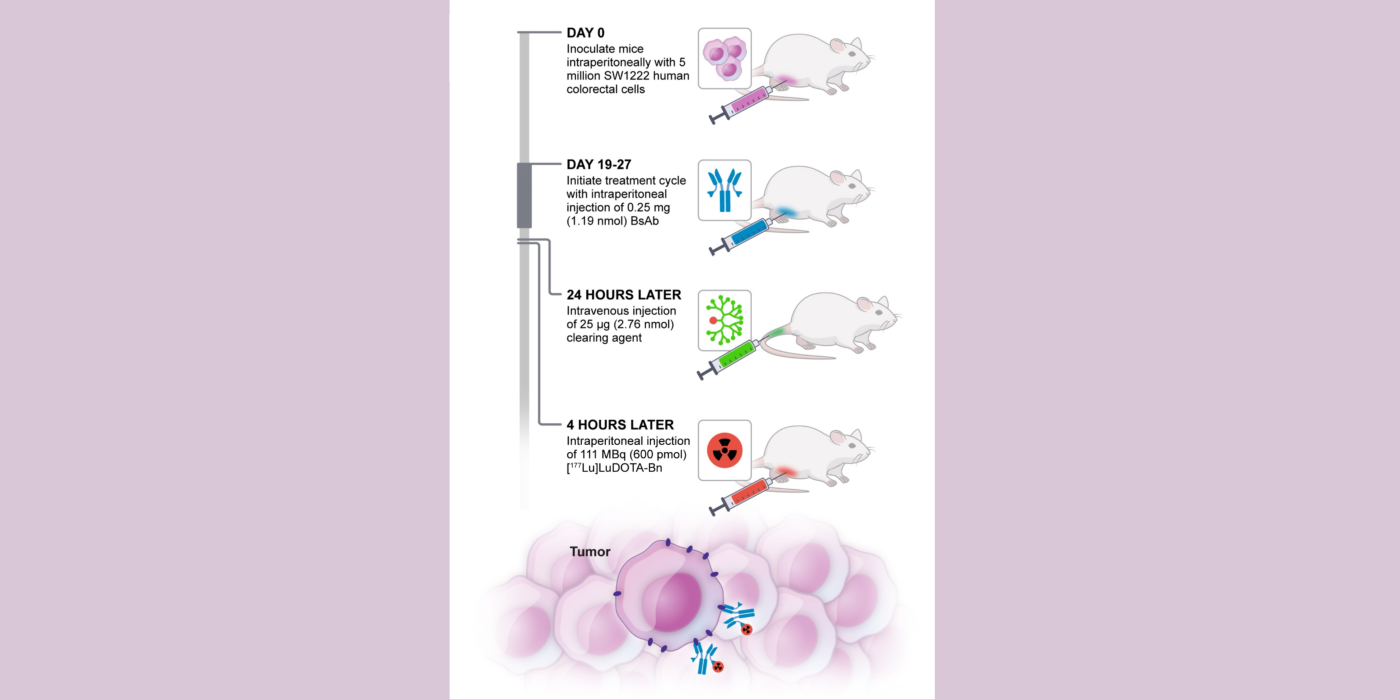
Fig 2. Nude mice bearing established GPA33-expressing SW1222 human colorectal xenografts (lower flank) undergoing GPA33-targeted DOTA-PRIT with a 177Lu-DOTA-radiohapten. GPA33 is a cell-surface marker expressed on >95% of colon cancers. 177Lu has both beta particle emissions for therapy and gamma emissions for imaging. SPECT/CT images were performed at 24 hours post-injection of 177Lu-DOTA-radiohapten using a dedicated small-animal SPECT/CT scanner. Based on image analysis, the 177Lu-uptakes in tumor and kidney were 9.42 %ID/g and 0.72 %ID/g, respectively, giving a tumor-to-kidney activity ratio of 14. Collectively, these data suggest highly favorable TIs during therapy. PMID: 28705917
See Release
We have also extended the approach to actinium-225 (225Ac), an alpha-emitting isotope. Please see PMID: 33052220
See Release
We recently applied this approach to treat aggressive intraperitoneal ovarian cancer with 225Ac-radiohapten. Please see PMID: 37348919
See Release
Sponsored by Y-mAbs Therapeutics, Inc. the SADA platform + 177Lu-DOTA is currently being investigated in a phase I clinical trial for the treatment of certain GD2-positive solid tumors, including small cell lung cancer, sarcoma and malignant melanoma. Please see NCT05130255
See Release
Bugs as drugs: harnessing engineered bacterial therapies to overcome limitations of conventional human tumor antigens for radiopharmaceutical therapy (in collaboration with Neil Forbes PhD of University of Massachusetts Amherst)
Radiopharmaceutical therapy (RPT) has incredible potential to be a first-line therapy for multiple cancer indications. During RPT, a therapeutic radionuclide is localized specifically in malignant tissue to directly deliver ionizing radiation. To enhance patient-specific dosimetry and treatment planning, whole-body molecular imaging (PET or SPECT) can be used to assess the extent of tumor targeting. Despite its enormous promise, the theranostic RPT approach has produced objective responses in only 30-60% of patients. In solid tumors, the potential for cures is limited by the low therapeutic indices (TIs) achievable with common targeting vectors. Engineered bacterial therapies have extraordinarily high tumor-to-normal tissue accumulation ratios without the requirement of a tumor-specific surface antigen target (i.e., epitope-independent tumor-tropism), especially to key radiosensitive issues that are dose-limiting (red bone marrow and kidney).
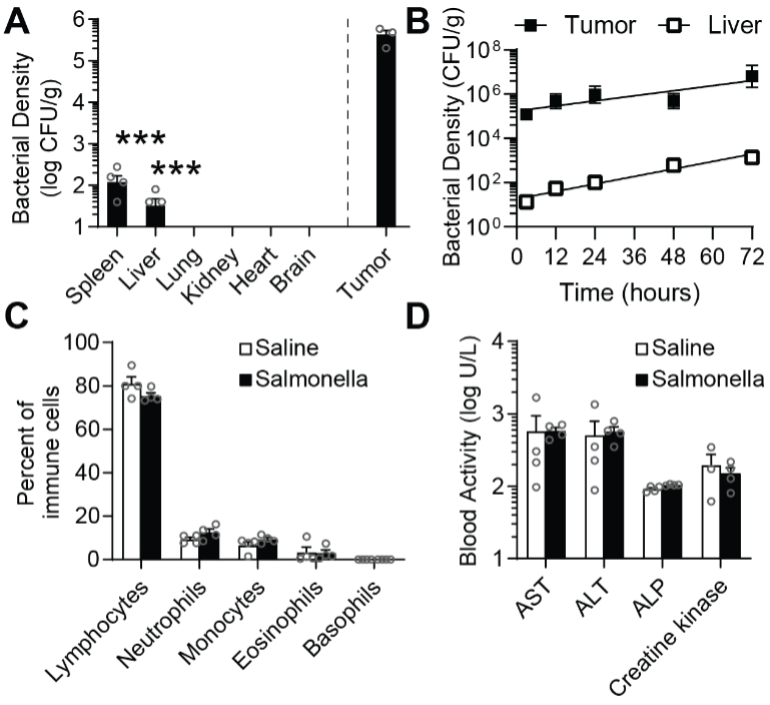
Fig 3. Salmonella are safe and preferentially accumulate in tumors. A) Biodistribution in BALB/c mice, 14 d after IV injection of 1×107 Salmonella. Bacterial densities in the spleen and liver were 3000 times lower than in tumors (***, P = 0.0001; n = 5). Densities were below detection in lungs, kidneys, hearts and brains. This accumulation pattern is the basis for high therapeutic index. B) As soon as three hours after injection, bacteria preferentially accumulated in tumors over livers. The relative density grew to 4,600 by 72 h. C,D) Comprehensive hematology (C) and chemistry profiling (D) showed no differences in the number of immune cells and no liver damage (ALP, alkaline phosphatase; ALT, alanine transaminase; and AST, aspartate transaminase) compared to saline controls.
Our central hypothesis is that tumortropic Salmonella can be genetically engineered with bioorthogonal markers for imaging and therapy.
Immuno-positron emission tomography (immunoPET) for non-invasive interrogation of tumor biology and patient selection for biomarker-targeted therapy (in collaboration with Scott Tagawa MD, Joseph Osborne MD PhD, James Kelly PhD, and Edward Fung PhD of Weill Cornell Medicine).
ImmunoPET combines highly specific monoclonal antibodies (mAb) with PET imaging (frequently the radiometal 89Zr, t½ = 3.3 days). As evidenced by the dozens of clinical-stage probes, immunoPET is emerging as a valuable molecular imaging tool in oncology. For the imaging and treatment of advanced prostate cancer, the humanized mAb J591, which binds prostate-specific membrane antigen (PSMA) on the surface of epithelial prostate cancer, has been used as a carrier for radionuclides (for immunoPET: 89Zr-J591 or 124I-J591; for radioimmunotherapy: 177Lu-J591 or 225Ac-J591) and cytotoxic drugs (maytansinoid DM1 or monomethyl auristatin E).
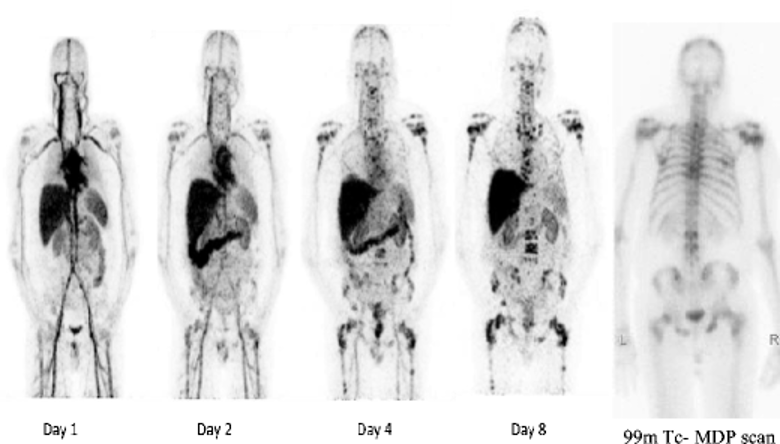
Fig 4. 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Serial whole-body 89Zr-huJ591 scans (MIP images). Images show physiological distribution in cardiac and vascular blood pool, decreasing with time, in liver, spleen, kidneys, and GI tract. Images show increased accumulation in multiple bone lesions, best seen in day 8 image. Many of these lesions are not clearly visualized on 99mTc-MDP scan (right). PMID: 25143071
We strive to develop novel immunoPET agents for advanced prostate cancer, with an emphasis on biomarker targets alternative to PSMA and diagnostic surrogates for radioimmunotherapy with 225Ac-huJ591.
List of therapeutic and/or diagnostic imaging probes
(1) Bispecific antibody-based imaging probes (86Y, 111In; development ongoing: 68Ga, 89Zr) against GD2, HER2, GPA33 antigens. Other antigen targets reported: CD38, CD45, CD20, CEA.
(2) Bispecific antibody-based therapy probes (177Lu, 225Ac; development ongoing: 212Pb) against GD2, HER2, GPA33 antigens. Other antigen targets reported (90Y): CD38, CD45, and CD20.
(3) 89Zr-labeled monoclonal antibodies (in development: 89Zr-CEACAM5 mAb)
Grants
NIH R01 CA233896 (PI), NIH R37 CA262557 (co-I), WCM Prostate SPORE Career Enhancement Program Award November 2023-October 2024 (PI).
Publications
2023
- Kurtz K, Eibler L, Dacek MM, Carter LM, Veach DR, Lovibond S, Reynaud E, Qureshy S, McDevitt MR, Bourne C, Monette S, Punzalan B, Khayat S, Verma S, Kesner AL, Cheung, NV, Schöder H, Gajecki L, Cheal SM, Larson SM, Scheinberg DA, Krebs S. Engineering CAR-T cells for radiohapten capture in imaging and radioimmunotherapy applications. Theranostics (2023) Oct 8;13(15):5469-5482. PMID: 37908719
- Chung SK, Vargas DB, Chandler CS, Katugampola S, Veach DR, McDevitt MR, Seo SH, Vaughn BA, Rinne SS, Punzalan B, Patel M, Xu H, Guo HF, Zanzonico PB, Monette S, Yang G, Ouerfelli O, Nash GM, Cercek A, Fung EK, Howell RW, Larson SM, Cheal SM, Cheung, NV. Efficacy of HER2-Targeted Intraperitoneal 225Ac α-Pretargeted Radioimmunotherapy for Small-Volume Ovarian Peritoneal Carcinomatosis. J Nucl Med (2023) Sep;64(9):1439-1445. PMID: 37348919
2022
- Cheal SM, Chung SK, Vaughn BA, Cheung NV, Larson SM. Pretargeting: A Path Forward for Radioimmunotherapy. J Nucl Med (2022), Sep;63(9):1302-1315. PMID: 36215514
- Chandler C, Bell MM, Chung SK, Veach DV, Fung EK, Punzalan B, Burnes Vargas D, Patel MP, Xu H, Guo H, Santich BH, Zanzonico PB, Monette S, Nash GM, Cercek A, Jungbluth AA, Pandit-Taskar N, Cheung NV, Larson SM, Cheal SM. Intraperitoneal Pretargeted Radioimmunotherapy for Colorectal Peritoneal Carcinomatosis. Mol Cancer Ther (2022), 21(1),125-137. PMID: 34667111
2021
- Dacek MM, Veach DR, Cheal SM, Carter LM, McDevitt MR, Punzalan B, Burnes Vargas D, Kubik TZ, Monette S, Santich BH, Yang G, Ouerfelli O, Kesner AL, Cheung NV, Scheinberg DA, Larson SM, Krebs S. Engineered Cells as a Test Platform for Radiohaptens in Pretargeted Imaging and Radioimmunotherapy Applications. Bioconjug Chem (2021), 32(4), 649-654. PMID: 33819023
- Santich BH, Cheal SM, Ahmed M, McDevitt MR, Ouerfelli O, Yang G, Veach DR, Fung EK, Patel M, Burnes Vargas D, Malik AA, Guo HF, Zanzonico PB, Monette S, Michel AO, Rudin CM, Larson SM, Cheung NK. A Self-Assembling and Disassembling (SADA) Bispecific Antibody (BsAb) Platform for Curative Two-step Pretargeted Radioimmunotherapy. Clin Cancer Res 2021, 27(2):532-541. PMID: 32958698
2020
- Cheal SM, McDevitt MR, Santich BH, Patel M, Yang G, Fung EK, Veach DR, Bell M, Ahad A, Vargas DB, Punzalan B, Pillarsetty N, Xu H, Guo HF, Monette S, Michel AO, Piersigilli A, Scheinberg DA, Ouerfelli O, Cheung NV, Larson SM. Alpha radioimmunotherapy using 225Ac-proteus-DOTA for solid tumors-safety at curative doses. Theranostics 2020, 10(25),11359-11375. PMID: 33052220
- Cheal SM, Patel M, Yang G, Veach D, Xu H, Guo HF, Zanzonico PB, Axworthy DB, Cheung NV, Ouerfelli O, Larson SM. An N-Acetylgalactosamino Dendron-Clearing Agent for High-Therapeutic-Index DOTA-Hapten Pretargeted Radioimmunotherapy. Bioconjug Chem (2020), 31(3), 501-506. PMID: 31891487
2019
- O'Donoghue JA, Danila DC, Pandit-Taskar N, Beylergil V, Cheal SM, Fleming SE, Fox JJ, Ruan S, Zanzonico PB, Ragupathi G, Lyashchenko SK, Williams SP, Scher HI, Fine BM, Humm JL, Larson SM, Morris MJ, Carrasquillo JA. Pharmacokinetics and Biodistribution of a [89Zr]Zr-DFO-MSTP2109A Anti-STEAP1 Antibody in Metastatic Castration-Resistant Prostate Cancer Patients. Mol Pharm (2019), 16(7), 3083-3090. PMID: 31117485
- Carrasquillo JA, Fine B, Pandit-Taskar N, Larson SM, Fleming S, Fox JJ, Cheal SM, O'Donoghue JA, Ruan S, Ragupathi G, Lyashchenko SK, Humm JL, Scher HI, Gonen M, Williams S, Danila DC, Morris MJ. Imaging metastatic castration-resistant prostate cancer patients with 89Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med (2019), 60(11), 1517-1523. PMID: 31053681
2018
- Cheal SM, Xu H, Guo HF, Patel M, Punzalan B, Fung EK, Lee SG, Bell M, Singh M, Jungbluth AA, Zanzonico PB, Piersigilli A, Larson SM, Cheung NV. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of HER2-positive breast carcinoma. Theranostics (2018), 8(18), 5106-5125. PMID: 30429889
- Cheal SM, Ruan S, Veach DR, Longo VA, Punzalan BJ, Wu J, Fung EK, Kelly MP, Kirshner JR, Giurleo JT, Ehrlich G, Han AQ, Thurston G, Olson WC, Zanzonico PB, Larson SM, Carrasquillo JA. ImmunoPET Imaging of Endogenous and Transfected Prolactin Receptor Tumor Xenografts. Mol Pharm (2018), 15(6), 2133-2141. PMID: 29684277