Areas of Investigation
Intracardiac Blood Flow Patterns in Murine Cardiac Disease Models
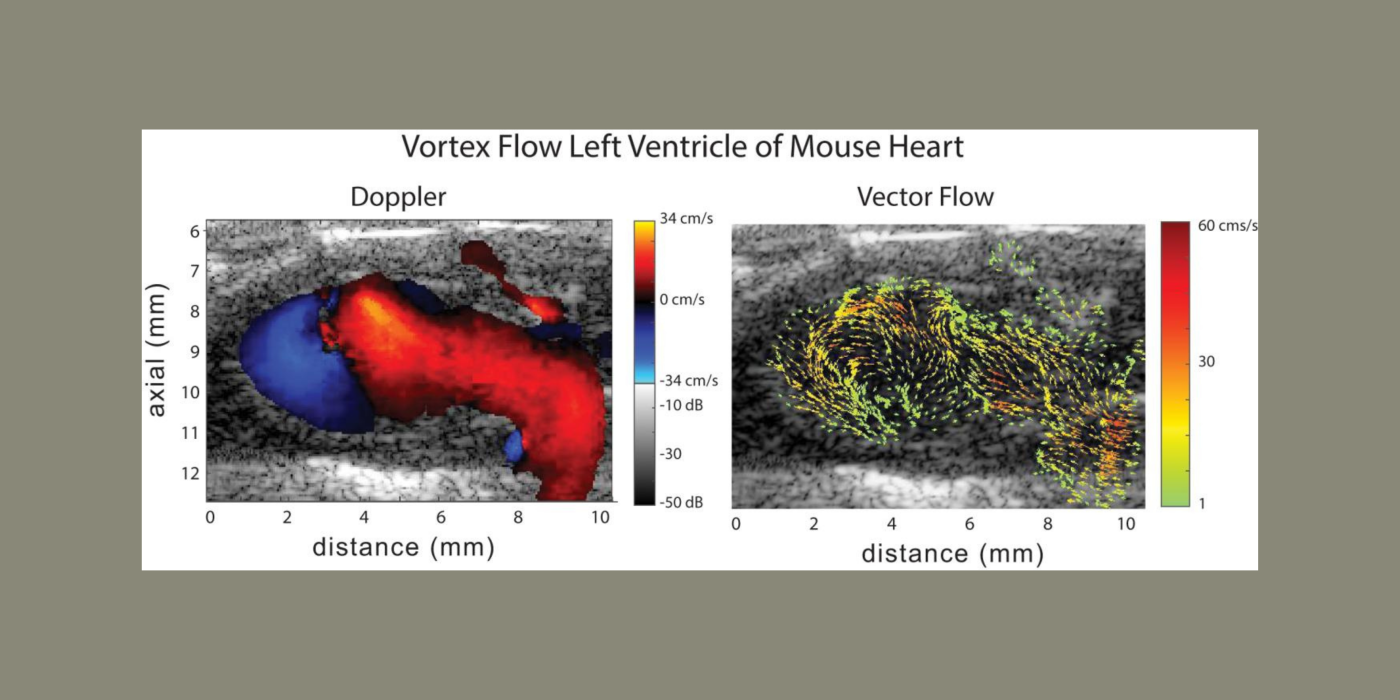
Cardiovascular disease (CVD) accounts for one of every three deaths each year in the U.S. Mice are the most common model organism for translational CVD studies of the mammalian heart. Ultrasound is now extensively used in small animals to obtain cardiac functional parameters. However, advanced ultrasound (US) intracardiac vector-flow imaging techniques that are gaining traction for human CVD, such as cardiomyopathies, have yet to translate to preclinical use, thus limiting the functional cardiac parameters that can be obtained from mice.
This project, in partnership with Dr. Oralkan at North Carolina State University, and Drs. Fishmann and Phoon at New York University (NYU) School of Medicine, seeks to develop a 30-MHz, 2D-capacitive micromachined ultrasonic transducer (CMUT), row-column (RC) high-frequency-ultrasound array, and a plane-wave vector-flow imaging approach capable of sub-ms, full-frame image capture for intracardiac imaging in mice. The CMUT array will let us collect data in adjacent planes to provide a 3D view of flow dynamics within the murine heart. We will study intracardiac left ventricle (LV) blood flow patterns in two highly related mouse strains that display divergent responses (progressive hypertrophy vs. dilatation and failure) to abnormal pressure overload. We seek to quantify abnormal left ventricle flow patterns relative to sham control mice, and to detect flow disruption prior to changes in traditional functional echo or strain measures. The ability to detect subtle phenotypic changes in common mouse models of CVD that are a result of early-stage diseases, or therapies, may translate to earlier and more aggressive treatment of patients at highest risk of pressure-overload induced heart failure.
Vitreo-retinal disease imaging with 3D annular-array ultrasound
The goal of this project is to implement quantitative ultrasound (QUS) capabilities into a clinical ophthalmic ultrasound system to characterize vitreous inhomogeneity (i.e., clinically significant vitreous floaters called vision degrading myodesopsia) as it relates to states of health and disease. Age-related changes due to collagen cross-linking and aggregation with liquefaction create inhomogeneities that appear non-uniformly throughout the vitreous body. For patients with myopia, these processes occur earlier in life, when vitreo-retinal adhesion is still strong. They destabilize the vitreous body before adhesion to the retina is weakened, resulting in a variety of conditions that impact vision. The ability to depict vitreo-retinal organization will offer unique early-stage detection, and assessment of vitreo-retinal disease, in patients with myopia at risk for retinal detachment and vitreo-maculopathies resulting from traction.
In collaboration with Quantel Medical, the VMR Institute (Dr. Jerry Sebag), and Columbia University Irving Medical Center (Dr. Ronald Silverman) we seek to incorporate a new 3D probe and QUS capabilities into a state-of-the art, 20-MHz annular-array-based clinical ophthalmic ultrasound system. The initial phase of the project emphasized device enhancement processing of the ultrasound data and collection of age-normal and myopic patient data. The later stages of the project will focus on development and integration of a probe capable of volumetric acquisition, further patient data collection, and QUS classification methods that take advantage of the new volumetric data. The final system will permit quantitative characterization of the vitreous body and allow for data-based treatment decisions of vitreo-retinal diseases.
WCM
Outside
- Orlando Aristizabal, M.S.
- Glenn Fishman, M.D.
- Donny Hoang, M.D.
- Omer Oralkan, Ph.D.
- Colin Phoon, M.D.
- Jerry Sebag, M.D.
- Ronald Silverman, Ph.D.
- Tadashi Yamaguchi, Ph.D.