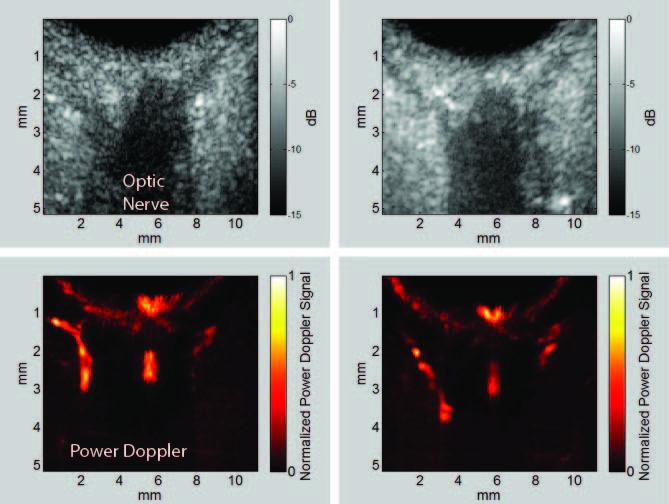
Glaucoma is an optic neuropathy and the leading cause of blindness, affecting over 3 million people in the United States. Elevated intraocular pressure (IOP) is a well-known risk factor for this disease, but reduced ocular perfusion is also now appreciated to be an important risk factor in primary open-angle glaucoma (POAG), the most common form of glaucoma. While optical methods such as optical coherence tomography (OCT) have made great strides in imaging the retina, its constituent layers (including the all-important nerve fiber layer in glaucoma) and recently even the choroid and lamina cribrosa, OCT penetration is limited beyond a 1 mm depth. OCT cannot adequately penetrate the anterior sclera for imaging or assessment of perfusion in the ciliary body, whose epithelium is responsible for aqueous fluid production and is hence the target for some medical treatments for control of IOP. OCT cannot image the orbital vessels supplying the eye. Although ultrasound has lower resolution than OCT, it has far greater penetration. High-frequency ultrasound can visualize the ciliary body in detail while Doppler studies at lower frequencies enable assessment of orbital blood flow. A major impediment to the adoption of ultrasound for ocular blood-flow imaging has been the stringent exposure criteria established by the Food and Drug Administration, the most conservative for any organ, including pre-natal scanning. Recently, a linear array-based ultrasound technique called coherent compound plane-wave imaging has been developed that offers not only an enormous increase in image frame rate, but also allows imaging of anatomy and perfusion at significantly reduced exposure intensities. To better understand the link between glaucoma and perfusion and, ultimately, develop methods that could have a significant clinical impact, we seek to utilize coherent compound plane-wave imaging to assess perfusion in POAG. We will develop and utilize this technology to assess orbital blood supply (at 10 MHz), choroidal and optic nerve perfusion (at 20 MHz) and the ciliary body (at 30 MHz). We will use the Verasonics Vantage 128 imaging engine, which is user-programmable, to produce anatomic and color-flow images in conjunction with measurement of IOP and retinal nerve fiber layer and ganglion cell complex thickness distributions and optic nerve head measures obtained by OCT and visual fields. Perfusion in each region will be compared between POAG and age-matched normal subjects. We will also characterize perfusion, especially in the ciliary body, pre- and post- medical therapy. Regional perfusion will be assessed statistically as a risk factor for progression of visual field loss. At the end of this project, we will have a better understanding of the role of perfusion in POAG, and have developed a clinically safe, acceptable, and diagnostically significant system for assessment of glaucoma with ultrasound-derived perfusion imaging of the choroid, optic nerve, orbital vessels, and ciliary body. Glaucoma is an optic neuropathy and the leading cause of blindness, affecting over 3 million people in the United States. Elevated intraocular pressure (IOP) is a well-known risk factor for this disease, but reduced ocular perfusion is also now appreciated to be an important risk factor in primary open-angle glaucoma (POAG), the most common form of glaucoma. While optical methods such as optical coherence tomography (OCT) have made great strides in imaging the retina, its constituent layers (including the all-important nerve fiber layer in glaucoma) and recently even the choroid and lamina cribrosa, OCT penetration is limited beyond a 1 mm depth. OCT cannot adequately penetrate the anterior sclera for imaging or assessment of perfusion in the ciliary body, whose epithelium is responsible for aqueous fluid production and is hence the target for some medical treatments for control of IOP. OCT cannot image the orbital vessels supplying the eye. Although ultrasound has lower resolution than OCT, it has far greater penetration. High-frequency ultrasound can visualize the ciliary body in detail while Doppler studies at lower frequencies enable assessment of orbital blood flow. A major impediment to the adoption of ultrasound for ocular blood-flow imaging has been the stringent exposure criteria established by the Food and Drug Administration, the most conservative for any organ, including pre-natal scanning. Recently, a linear array-based ultrasound technique called coherent compound plane-wave imaging has been developed that offers not only an enormous increase in image frame rate, but also allows imaging of anatomy and perfusion at significantly reduced exposure intensities. To better understand the link between glaucoma and perfusion and, ultimately, develop methods that could have a significant clinical impact, we seek to utilize coherent compound plane-wave imaging to assess perfusion in POAG. We will develop and utilize this technology to assess orbital blood supply (at 10 MHz), choroidal and optic nerve perfusion (at 20 MHz) and the ciliary body (at 30 MHz). We will use the Verasonics Vantage 128 imaging engine, which is user-programmable, to produce anatomic and color-flow images in conjunction with measurement of IOP and retinal nerve fiber layer and ganglion cell complex thickness distributions and optic nerve head measures obtained by OCT and visual fields. Perfusion in each region will be compared between POAG and age-matched normal subjects. We will also characterize perfusion, especially in the ciliary body, pre- and post- medical therapy. Regional perfusion will be assessed statistically as a risk factor for progression of visual field loss. At the end of this project, we will have a better understanding of the role of perfusion in POAG, and have developed a clinically safe, acceptable, and diagnostically significant system for assessment of glaucoma with ultrasound-derived perfusion imaging of the choroid, optic nerve, orbital vessels, and ciliary body.