Urinary Disposing Peptide
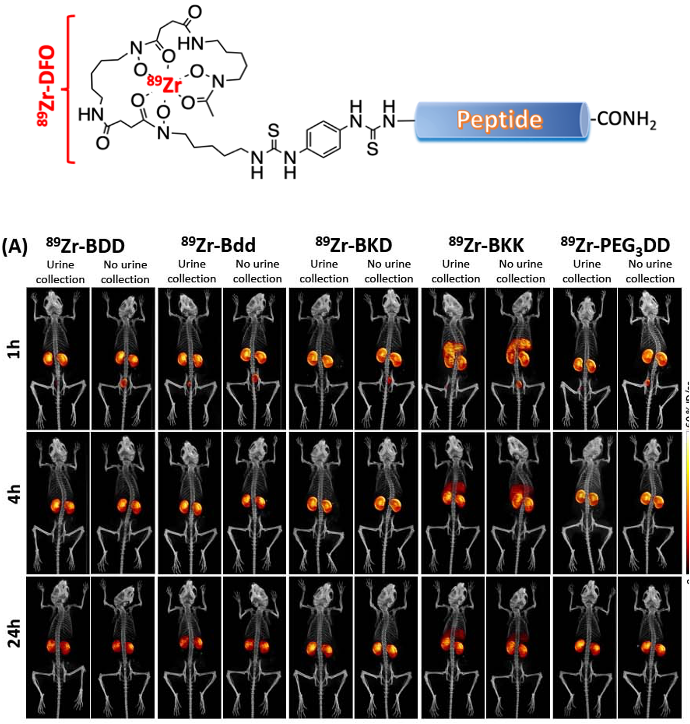
For a successful therapy, drug plasma concentration needs to be maintained at a certain level prior to reaching the treatment sites. Without chemical modifications, peptides display unfavorable PK (a very short circulating half-life <2 h). They can be rapidly degraded by protease and eliminated by renal filtration and secretion. We will use a peptide’s “disadvantage” (rapid renal clearance) as an approach to direct (disposing) treatments to the URS. We used a biologically inert peptide, like Bdd, that can be eliminated exclusively via renal clearance with minimal non-specific uptake by other organs. This approach can be an alternative to ITC to overcome the delivery barrier of both NMIBC and MIBC treatments and potentially improve drug efficacy and patient compliance.
Peptide-based nanofibers as imaging platform
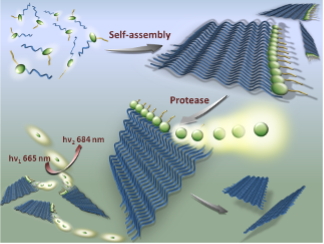
Abnormal proteolysis is often observed during disease progression. Up-regulation of certain tumor-associated proteases such as urokinase plasminogen activator (uPA) can be a hallmark of malignant transformation. We developed a near-infrared nanofiber precursor for detecting uPA activity. The nanofiber is optically silent in its native state. On uPA activation, it releases peptide fragments that contribute to a significant fluorescence amplification at 684 nm that can useful for imaging the enzyme activity in vivo.
Nanofibers as drug delivery platform
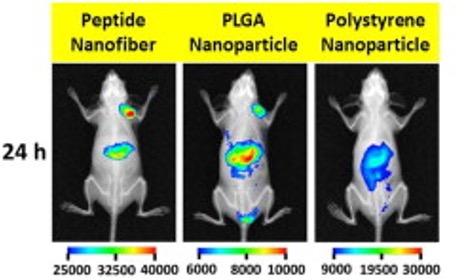
A small short circulating peptide-based drug carriers showing an effective tumor uptake by the enhanced permeability retention (EPR) effect with minimal uptake by the reticuloendothelial system (RES). We have employed such technology to deliver chemotherapeutics such as doxorubicin and DM1 for treatment of breast, brain, and bladder cancers.
Sequential-FRET imaging nanoprobes
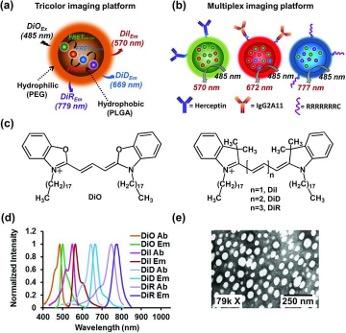
The ability to map multiple biomarkers at the same time has far-reaching biomedical and diagnostic applications. We have a series of biocompatible nanoparticles for multicolor and multiplexed imaging. An advantage of using FRET is the low background signal, given to a larger gap between the excitation and emission maxima. These particles can be decorated with with specific (Herceptin or IgG2A11 antibody) or nonspecific (heptaarginine) ligands for targeting.
Kinase sensors
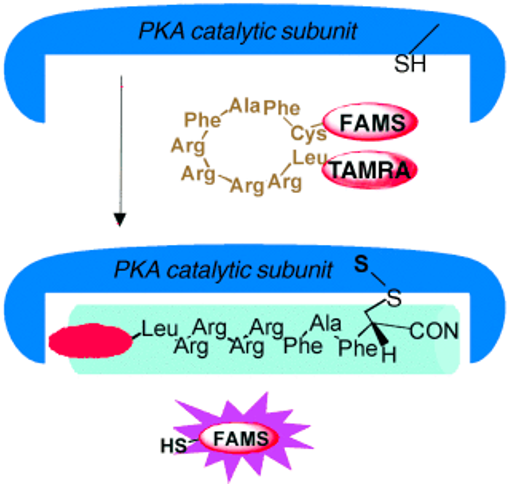
We introduced a mechanism-based fluorescent reporter designed for the detection of protein kinase A, such as PKA, which is known to mediate a variety of cellular responses in most eukaryotic cells. The probe consists of a specific binding peptide sequence, LRRRRFAFC, conjugated with fluorophores. Upon PKA addition, the reporter reacts with the sulfhydryl functionality through a disulfide-exchange mechanism, resulting in significant fluorescence amplification. The remaining peptide sequence, which also acts as an inhibitor, is attached covalently to the enzyme.
Peptide-based hydrogel as therapeutic implants
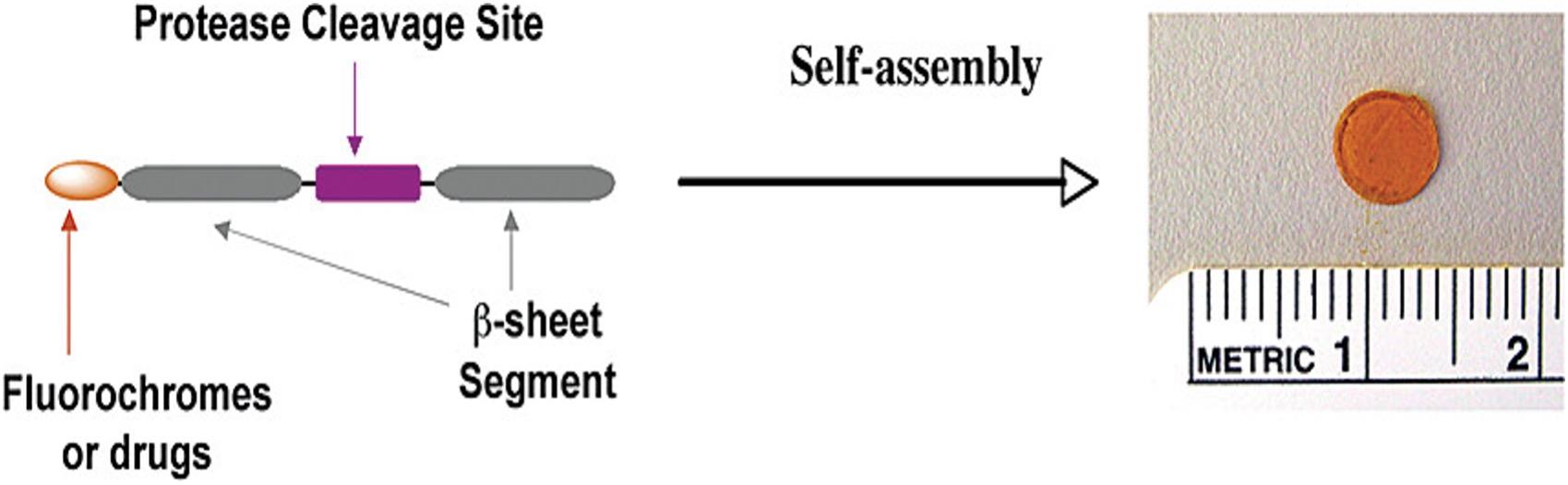
Controlled delivery of drugs in response to tumor-microenvironment has the potential of targeting therapies and personalized treatments. We have a developed a peptide-based hydrogel that can release therapeutic payloads upon specific interaction with tumor-associated proteases. The core peptide sequence is composed of a protease cleavable region flanked by β-sheet forming motifs. In aqueous solution, peptides self-assemble into a gel matrix. Therapeutic agents can be encapsulated into this formulation. Upon addition of the targeted protease, the enzyme digests the preparations at the substrate cleavage site, resulting in release of gel fragments and therapeutic agents.

