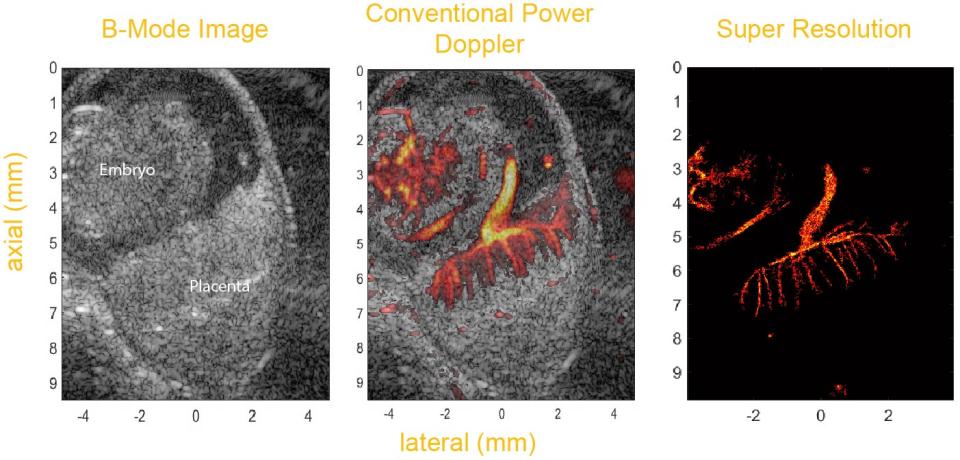
A fundamental question in developmental biology is how specific molecules and genetic pathways control morphogenesis during embryonic development. Recent studies have shown that many of the same molecules and genetic pathways affecting organogenesis are involved in vascular development and patterning during angiogenesis. A major challenge is to develop rapid, in vivo mouse-embryo imaging methods that can analyze organ and vascular patterning with fine resolution. The goal of this proposal is to establish the feasibility of transporting acoustic nanodrops (NDs) through the placenta to map vasculature of the embryonic mouse, in utero, with super-resolution, plane-wave ultrasonic imaging. Perfluorocarbon NDs can be vaporized by an acoustic excitation and converted to gas-filled acoustic contrast agents. After vaporization, the NDs reach a size on the order of 1 m and appear as bright points in an ultrasound image. We will formulate ND compositions of different size, charge, and perfluorocarbon core. We will evaluate fluorescent ND compositions to determine which NDs, after injection into the maternal mouse tail vein, pass through the placenta into the embryonic circulation and select the most promising composition in terms of size and transport efficiency. We will quantify the acoustic pressures at which the selected NDs vaporize. The most promising ND in terms of vaporization threshold will be injected into the maternal tail vein of a mouse and, after entering the embryonic circulation, the NDs will be activated with an acoustic pulse. Plane-wave ultrasound and super-resolution methods will then detect and localize activated NDs at fine spatial- and -temporal resolution as they move through the embryonic circulation. Feasibility of vascular mapping in a single plane will be evaluated. Because this approach relies on tracking point targets, the length scale that can be resolved, on the order of 20 m, is much less than the lateral beamwidth of the 18-MHz linear-array acoustic field. The ability to activate NDs that have passed through the placenta, and the ability to perform noninvasive, in-utero contrast-enhanced imaging, has high potential to revolutionize the way we study organogenesis and angiogenesis in widely utilized models of normal and abnormal embryonic development.