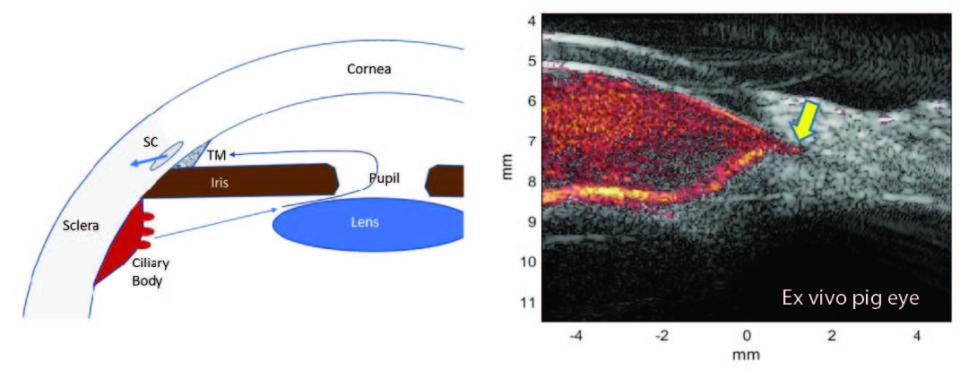
Glaucoma is one of the primary causes of irreversible vision loss in this country and worldwide. It is a multi-factorial disease, or family of diseases, characterized by death of retinal ganglion cells (RGCs) and optic neuropathy. It has long been known that age, elevated intraocular pressure (IOP), and family history are glaucoma risk factors. It is now appreciated that reduced ocular perfusion also represents a significant risk factor. At present, all medical and surgical approaches to glaucoma management focus on control of IOP. Potentially, correction of impaired ocular perfusion might be a fruitful approach in conjunction with conventional management, but to make progress in exploiting this factor, a means for evaluating flow in small animal models of glaucoma will be crucial. We developed ultrafast compound plane-wave ultrasound technology for visualization and measurement of flow in the orbital vessels, and choroid and anterior segments of the normal and glaucomatous human eye. There are many advantages, however, in applying this technique to animal models of glaucoma, where controlled experimental conditions and histologic evaluation can be utilized. The level of resolution provided by the plane-wave technique, however, is inadequate for small rodent models.
In the study, we will use the recently developed technique of super-resolution imaging (SRI) to address this shortcoming. SRI is based on tracking of contrast microbubbles that are much smaller than a wavelength as they move through the microvasculature. SRI, in combination with ultrafast plane wave imaging, will be used to image bloodflow in the orbital arteries, and choroid and anterior segments of the rat eye at non-diffraction limited, sub-wavelength resolution. We will characterize flow after acute elevation of IOP by anterior chamber cannulation by chronic IOP elevation induced by impairment of aqueous outflow produced by injection of hypertonic saline into the episcleral veins, and with optic nerve ischemia induced by laser photocoagulation of vessels at the optic nerve head. We will determine the effect of these interventions on ocular blood flow in the major vessels supplying the eye, and the choroid and anterior segments simultaneously. Time-lags between arterial and choroidal flow will be considered an indicator of uveal compliance, which may be altered in glaucoma. Measures of cumulative IOP and blood flow impairment will be correlated with RGC and optic nerve damage determined histologically. We will also treat ocular hypertensive rats with betaxolol to lower IOP, and with astaxanthin to enhance choroidal flow, and measure and compare their effects on IOP, flow, and tissue damage. The proposed research will demonstrate a new technique for characterization of orbital and uveal flow, enabling use of preclinical models for exploring the effect of improved ocular perfusion on glaucomatous neuropathy.