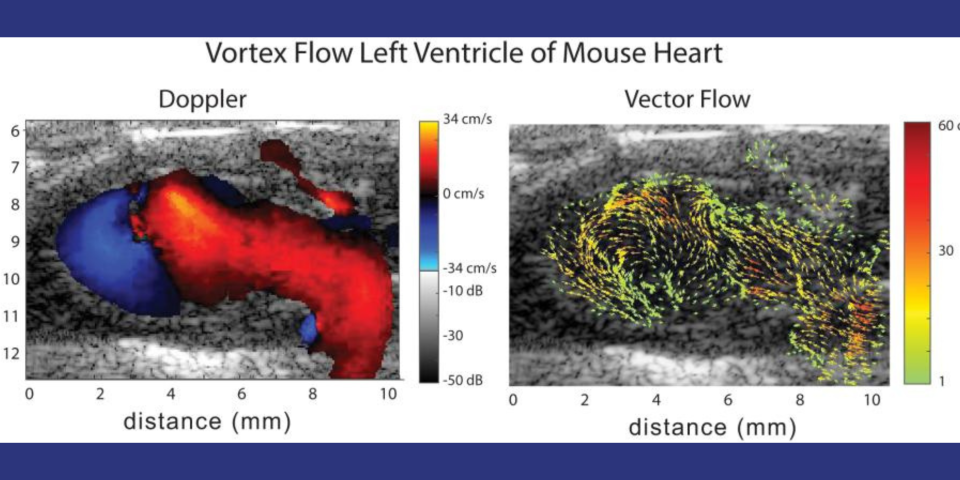
Cardiovascular disease (CVD) accounts for one of every three deaths each year in the U.S. A substantial proportion of patients with CVD develop myocardial dysfunction. Imaging tools that permit early detection of abnormal hemodynamics and/or mechanics provide an opportunity to initiate targeted therapeutics and diminish the burden of disease. Mice are the most common model organism for translational CVD studies of the mammalian heart. Ultrasound (US) is now extensively used in small animals to obtain cardiac functional parameters. However, advanced US intracardiac vector-flow imaging techniques that are gaining traction for human CVD, such as cardiomyopathies, have yet to translate to preclinical use, thus limiting the functional cardiac parameters that can be obtained from mice. The ability to employ US vector-flow methods to simultaneously resolve complex, intracardiac blood flow patterns and cardiac mechanics at sub-millisec temporal resolution, prior to overt structural and functional abnormalities, would add a new preclinical tool to study the interplay between blood flow, cardiac mechanics, and adaptation in CVD mouse models. The goal of this project is to develop a novel, 30-MHz, 2D CMUT, row-column (RC) high-frequency-ultrasound array and a plane-wave vector-flow imaging approach capable of sub-ms, full-frame image capture for intracardiac imaging in mice. Unlike a standard linear array, the 2D CMUT array will allow dynamic, hands-free selection of the optimal scan plane and the ability to acquire data in orthogonal image planes. In addition, the CMUT array will let us collect data in adjacent planes to provide a 3D view of flow dynamics within the murine heart. To validate our system and demonstrate the utility for small-animal imaging, we will study intracardiac left ventricle (LV) blood flow patterns in two highly related mouse strains that nonetheless display divergent responses (progressive hypertrophy vs. dilatation and failure) to abnormal pressure overload induced by the well-established model of transverse aortic constriction (TAC). We hypothesize that our vector-flow system will be able to quantify abnormal left ventricle flow patterns relative to sham control mice and that we will be able to detect flow disruption prior to changes in traditional functional echo or strain measures. Importantly, we also hypothesize that distinct flow pattern signatures can be identified early in the course of disease that will permit discrimination between hearts that are destined to develop progressive hypertrophy vs. dilation. The ability to detect subtle phenotypic changes in common mouse models of CVD that are a result of early-stage diseases or therapies may translate to earlier and more aggressive treatment of patients at highest risk of pressure-overload induced heart failure.