Areas of Investigation
Imaging tumor vascular properties
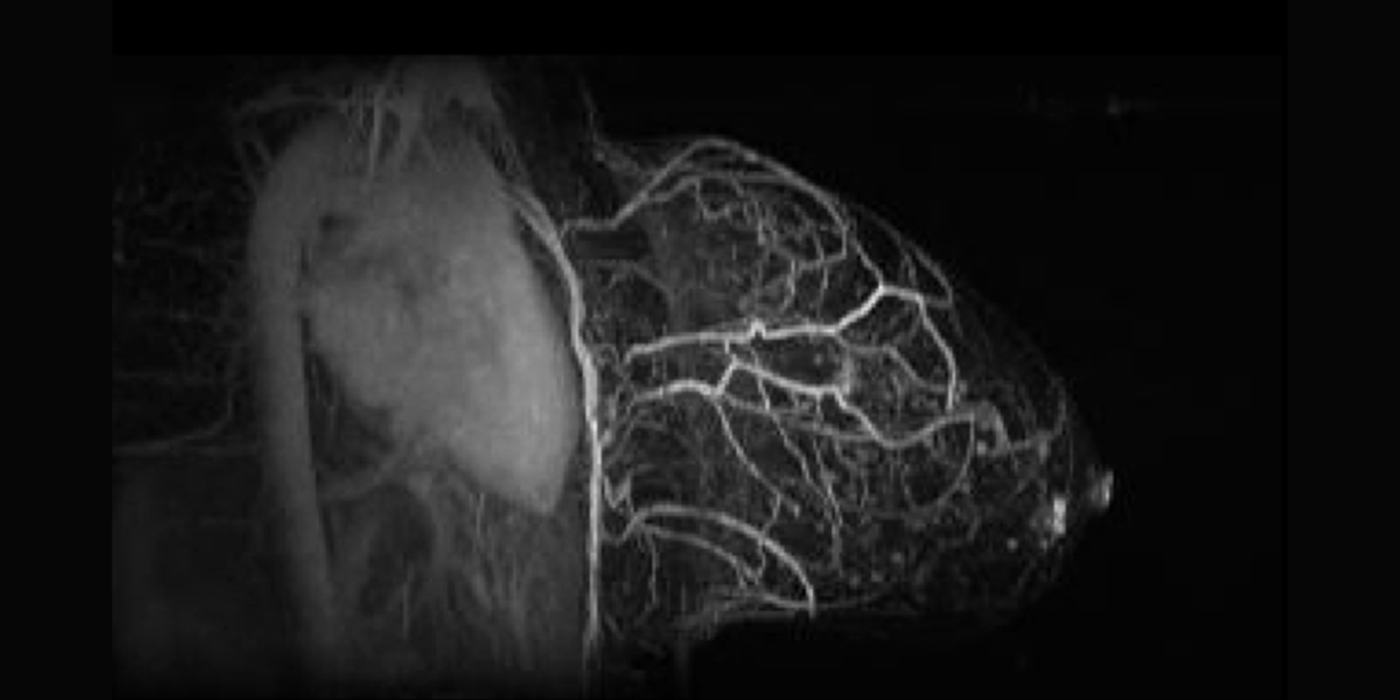
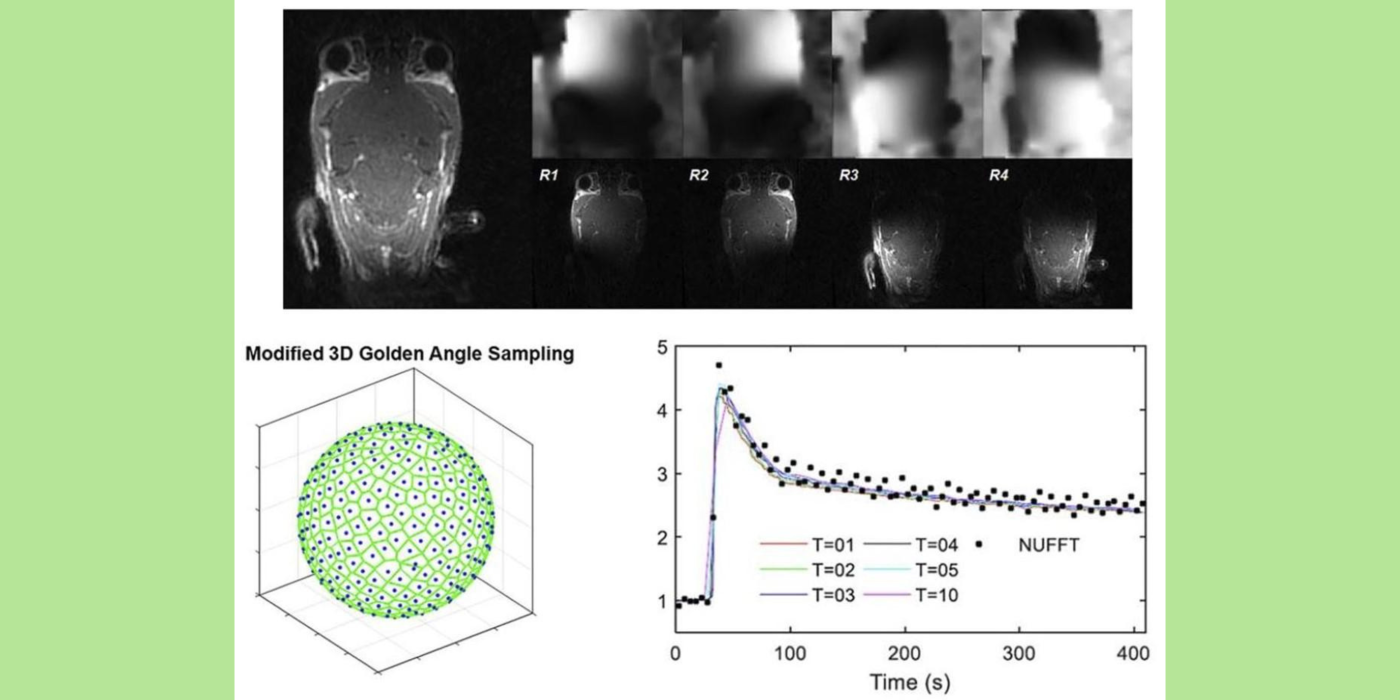
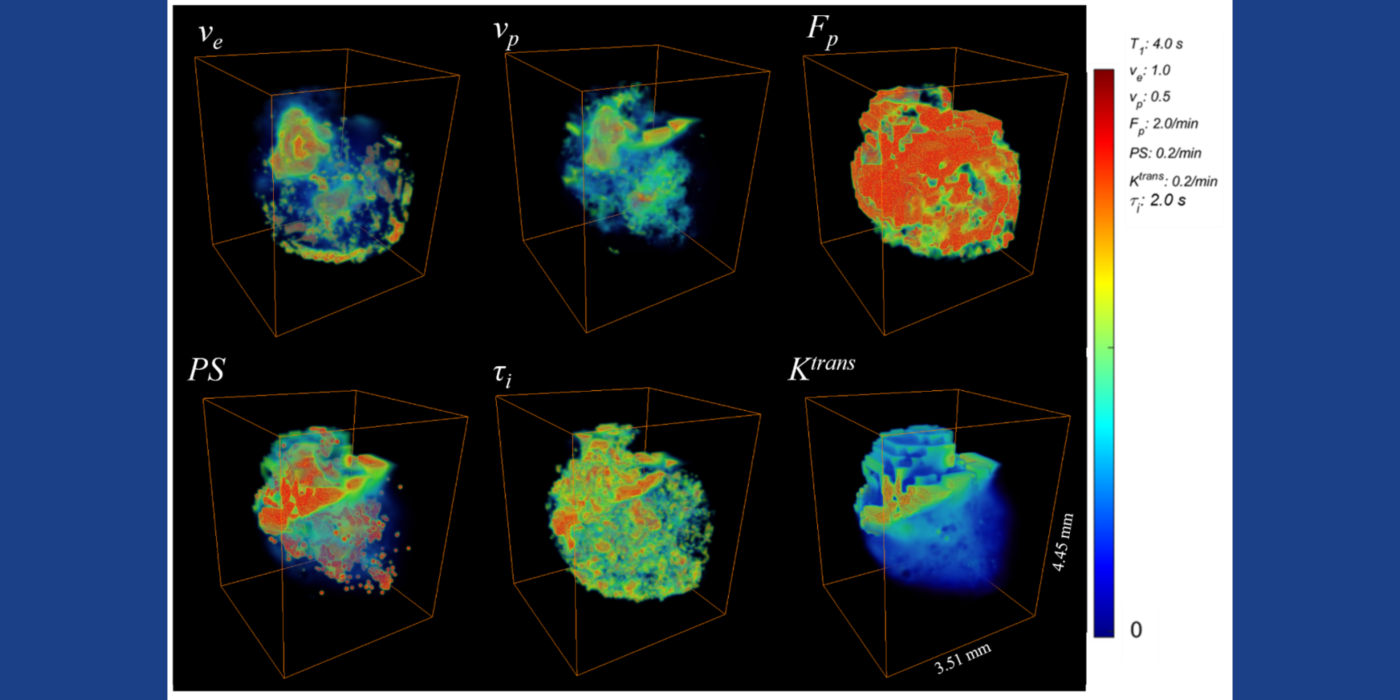
Chaotic vascular growth is a hallmark of malignant tumors and an important target for cancer treatment. The primary method that we have been using for the study of tumor vascularity is dynamic contrast enhanced (DCE) MRI (Kim et al., 2007 and 2010). In order to further understand the contrast dynamics inside the tumor with a high precision, the lab has been actively developing fast MRI methods that provide a higher temporal and spatial resolution, including the 3D ultra-short echo time (UTE) golden-angle radial sparse and parallel (GRASP) MRI method (Feng et al., 2014; Zhang et al., 2019).
Among many contrast kinetic parameters that can be measured using DCE-MRI, the Kim lab is particularly interested in the cellular-interstitial water exchange rate. The lab's recent head and neck cancer study found patients with slower transcytolemmal water exchange rates at pre-treatment have significantly prolonged overall survival at five years and beyond (Chawla et al., 2018). The lab showed that the effect of water exchange can be actively encoded in the dynamic data in order to improve the precision of water exchange rate measurement (Zhang and Kim, 2019). In addition, the lab introduced a single comprehensive imaging method, namely active contrast encoding (ACE)-MRI (Zhang et al., 2017), that provides pre-contrast T1 and actual flip angle from the dynamic data and eliminates the need to measure them separately. The goal: bring quantitative DCE-MRI to routine clinical imaging exams for accurate assessment of cancer treatment response, and also to extend its use to other microvascular diseases.
Imaging tumor cellular properties
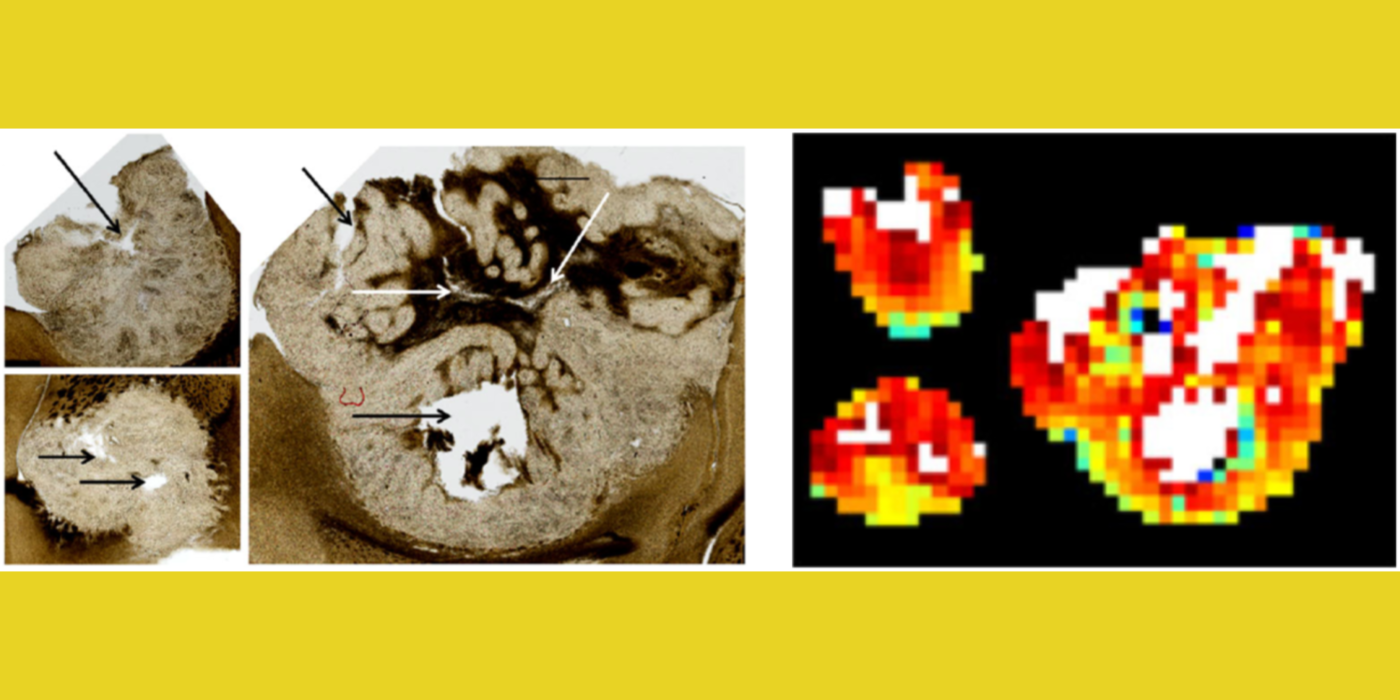
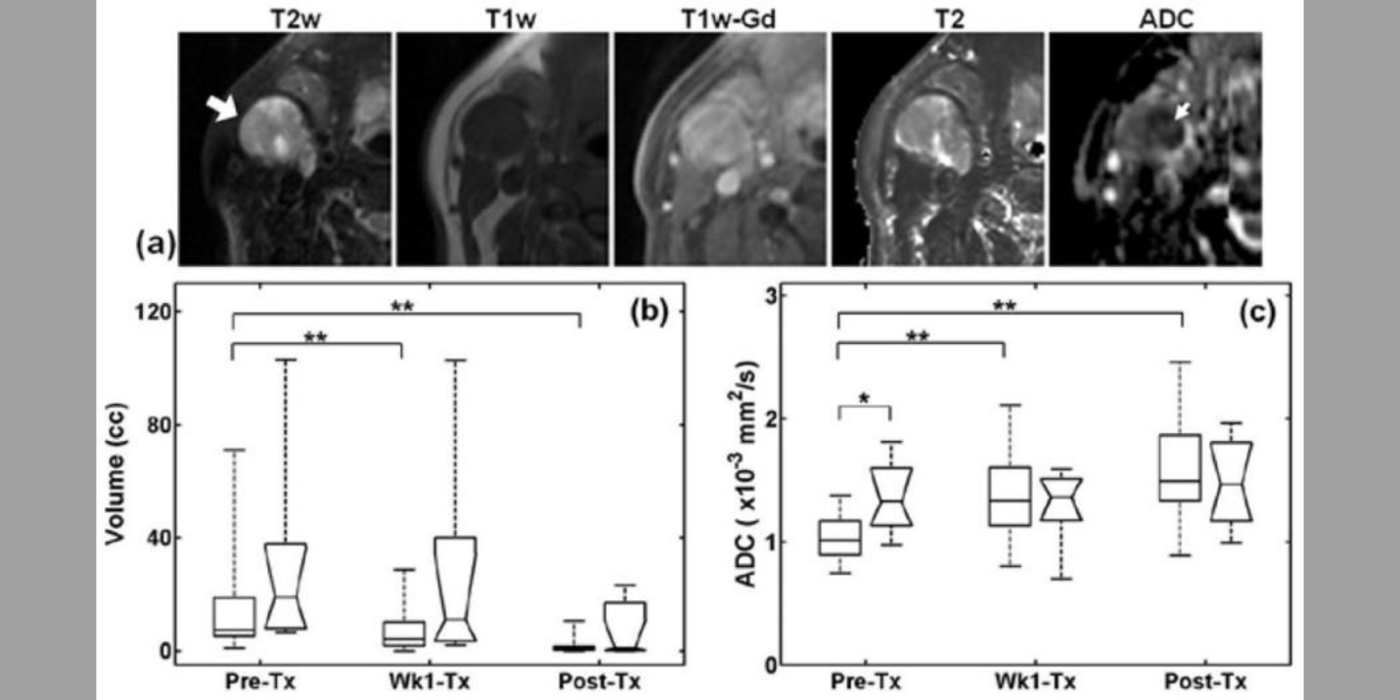
Another useful approach for the assessment of tumor treatment response is to measure cellular microstructural properties using diffusion MRI (dMRI) (Kim et al., 2009). Conventional dMRI measures such as apparent diffusion coefficient, however, remain non-specific biomarkers. The dMRI technique allows one to represent different biophysical properties of tissue depending on the diffusion weighting strength (q) and diffusion time (t) used for the measurement. The feasibility of measuring cell size using the t-dependency of dMRI was reported earlier with reference to different muscle groups (Kim et al., 2005). Inspired by these results, the lab has focused on investigating quantitative ways to utilize both the q- and t-dependency of dMRI data for assessment of cell viability by measuring cell size, extracellular volume fraction, and cellular compartmental diffusivities. This led the lab to propose the POMACE (pulsed and oscillating gradient MRI for assessment of cell size and extracellular space) framework (Reynaud et al., 2016) as a non-invasive imaging method to measure cellular microstructural properties.
Moreover, the lab has demonstrated that t-dependency of diffusional kurtosis can also be used to measure the cellular-interstitial water exchange rate (Zhang et al., 2021). This innovative approach does not require the use of an exogenous contrast agent to measure the water exchange rate and can provide a non-contrast imaging marker for cellular metabolism. The lab's investigation of quantitative dMRI measures in cancer, including the cellular-interstitial water exchange rate in head and neck cancer patients undergoing radiation therapy, is conducted in collaboration with the National Institutes of Health/National Cancer Institute (NIH/NCI) Quantitative Imaging Network.
Association of adipose tissue with cancer development
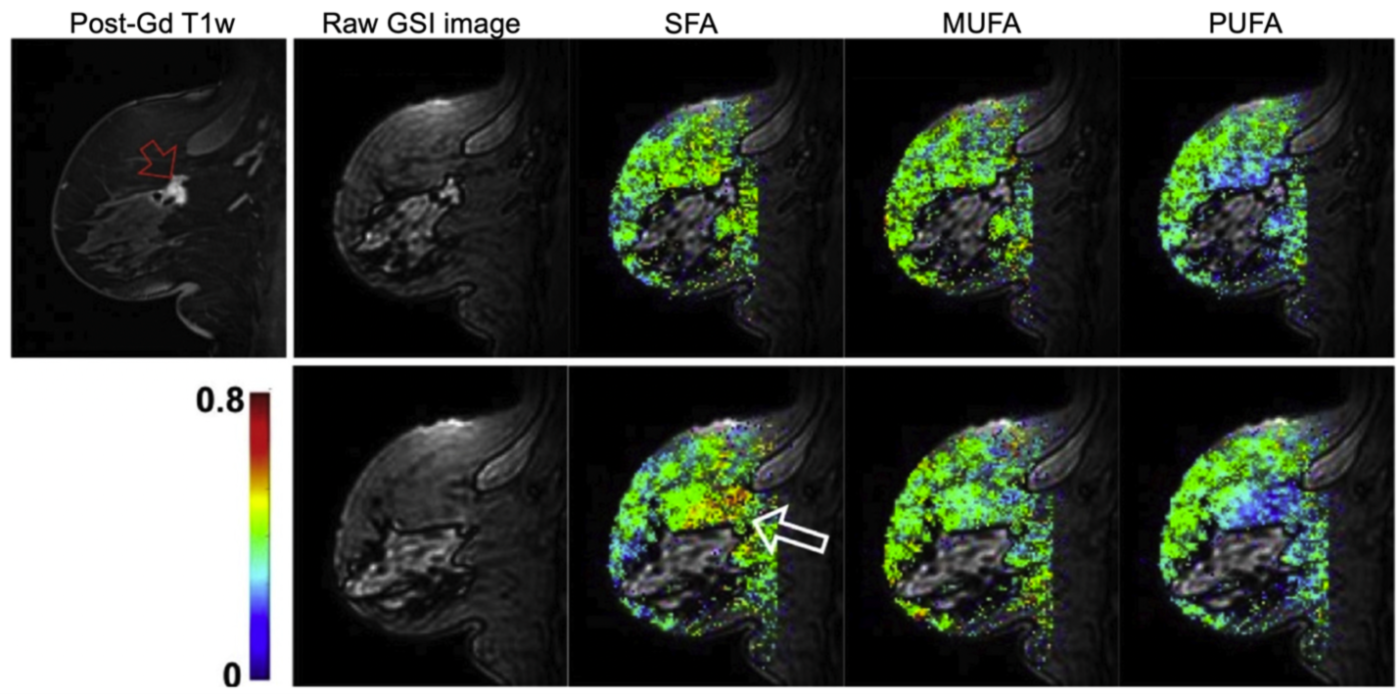
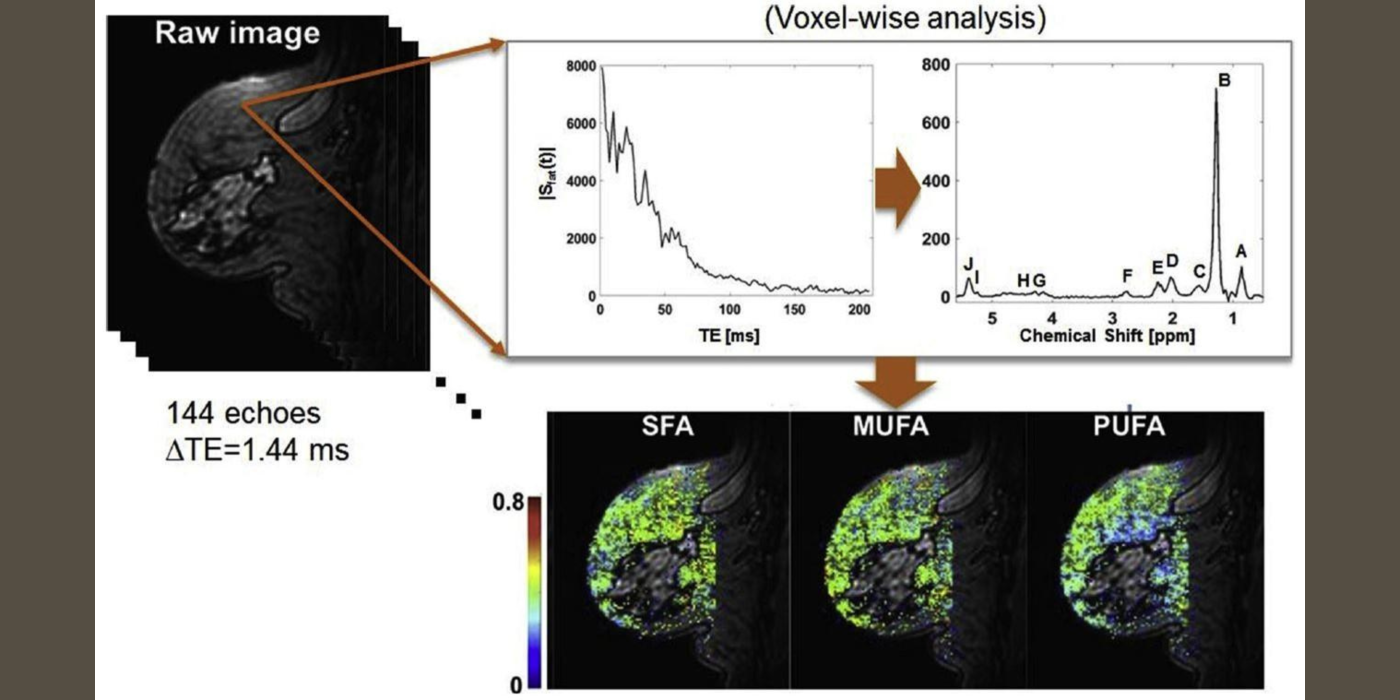
It has been increasingly recognized that adipose tissue may not be an “innocent bystander” in cancer development and progression. The lab has been developing fast MR spectroscopic imaging methods to measure fatty acid compositions in breast adipose tissue and investigating how these fatty acids are associated with breast cancer (Freed et al., 2016). After menopause, adipose tissue becomes the principal site of estrogen biosynthesis, and there is ample evidence demonstrating possible links between tissue hormone levels and fatty acid composition. While these hormonal effects are recognized as significant confounding factors in breast MRI, they may also provide a non-invasive means to study the effect of hormones on normal breast tissue and its association with breast cancer development and progression as demonstrated in our previous studies (Amarosa et al., 2013; Clendenen et al., 2013).
The team aims to develop MR spectroscopic imaging methods to measure fatty acid composition in breast adipose tissue and to assess its association with female hormones at the tissue level; the vascular and cellular properties of breast fibroglandular tissue; and cancer development.