Awards or Grants: UG3/UH3CA228699, National Cancer Institute NCI, 5/1/2019 to 4/30/2024
Description: Quantitative diffusion magnetic resonance imaging (dMRI) remains challenging as dMRI data represent different biophysical properties of tissue depending on diffusion weighting strength (q) and diffusion time (t) used for the measurement. The scientific premise of the study is that it will establish a quantitative way to utilize both q- and t-dependent dMRI data as a tailored approach to quantify cell viability, cellular metabolism, and perfusion via this non-contrast MRI method. Ultimately, these dMRI measures will better identify patients who have potential to benefit from adaptive de-escalation or escalation of therapy.
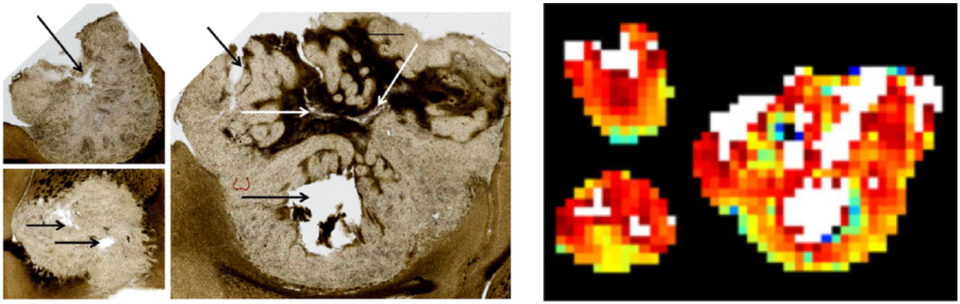
Project Summary: Chemo-radiation therapy is a standard treatment regimen for locally advanced head and neck squamous cell carcinoma (HNSCC). The treatment regimen, however, is difficult for patients, as they experience high rates of grade 3 or higher toxicities including leukopenia (42%) and the need for a feeding tube (52%). Recent studies showed a subgroup of HNSCC patients with human-papilloma virus (HPV)-positive oropharyngeal (OP) SCC have significantly better prognoses. These clinical data led to important considerations to de-intensify treatment for this low-risk, younger population to reduce acute and chronic toxicity without compromising disease control. It has been suggested the adaptive de-escalation of treatment can be tailored to individuals based on early tumor volume change. But volumetric assessment is often inadequate, as the treatment response of tumors can be heterogeneous in terms of (i) cell viability, (ii) cellular metabolism, and (iii) perfusion relevant to the success of chemoradiation therapy. These complex changes may not be adequately represented by early-stage tumor volume change.
This study is based on a combination of dMRI methods with technical innovations that can be applied to many clinical studies. The dMRI technique provides unique in vivo imaging sensitive to cellular microstructures at the scale of water diffusion length on the order of a few microns. But quantitative dMRI remains challenging as dMRI data represent different biophysical properties of tissue depending on diffusion weighting strength (q) and diffusion time (t) used for the measurement. Our scientific premise: this study will establish a quantitative way to utilize both q- and t-dependent dMRI data as a tailored approach to quantify cell viability, cellular metabolism, and perfusion from this non-contrast MRI method. We demonstrated that both diffusion coefficient D and diffusional kurtosis coefficient K are promising imaging markers for cell viability. Cellular metabolism can be evaluated via water exchange tex, measured by the diffusion time-dependent K, which is regulated by the adenosine triphosphate (ATP)-dependent trans-membrane ion channels co-transporting water molecules. Intravoxel incoherent motion MRI metrics (pseudo diffusivity, Dp; perfusion fraction, fp) can provide information about perfusion flow. Ultimately, these dMRI measures will better identify patients who have the potential to benefit from adaptive de-escalation or escalation of therapy.
In this study, the lab will further optimize and establish a set of quantitative non-contrast imaging markers of cell viability (D and K), cellular metabolism (tex), and perfusion (fp×Dp) as a clinical tool for assessment of treatment response, and validate it in a clinical trial. The data acquisition and analysis software tools to be developed in this study will enable comprehensive and quantitative assessment of cancer treatment response to tailor chemoradiation therapies for individual patients.