Award or Grant: National Heart Lung and Blood Institute (NHLBI), 1 R61 HL156153-01 (2021-2023)
Prior Associated Grant: Non-occluding Zero-Thickness Inflatable Sensor Arrays for Conformable Mapping and Ablation
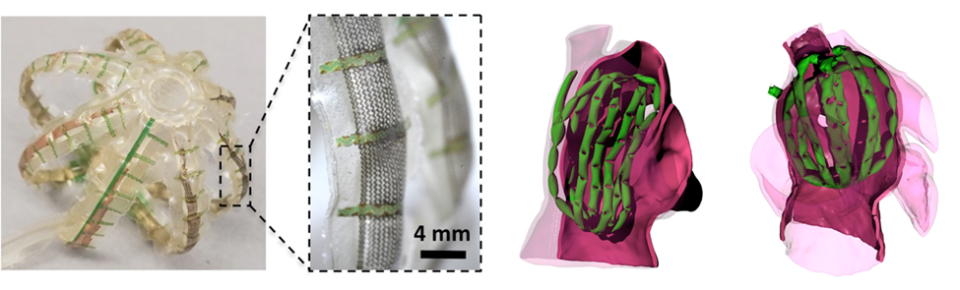
This project aims to develop and test a highly novel multi-electrode balloon catheter for acquiring whole chamber cardiac electrograms for atrial fibrillation (AF) with unprecedented fidelity and speed. In the large number of cases where pharmaceuticals cannot manage AF or generate severe side effects, minimally invasive electrodes are used to map electrical signals and ablate tissue thought to be the source of aberrant electrical pathways. While existing ablation protocols can treat the disease in many cases, these procedures can be lengthy (typically two to four hours) and are only somewhat effective (~60-70% success). These inefficiencies can be attributed in large part to a poor understanding of AF mechanisms. Complex spatio-temporal electrophysiological features, like “rotors,” play a key role in the disease, but typically cannot be detected by conventional mapping systems because their serial nature prevents measurements from being spatiotemporally resolved. New classes of multi-electrode (ME) catheters (known as basket catheters) were recently developed to simultaneously map the entire atrium. Here, an array of sensors are mounted on a spherical cage of stiff nitinol splines. The device is promising, but the rigid mechanical properties of the sensors and the frame they are mounted on yield poor conformability (~60% contact), diminishing performance. Additionally, sensor position is not well controlled; many sensors group in crevices in the atrial anatomy. So atrial maps often omit large regions of the chamber.
To address these challenges, Dr. Dunham and Dr. Mosadegh developed a cardiac mapping device consisting of collections of active, hydraulic, soft-robotic actuators integrated with arrays of flexible electronic sensors that can perform mapping of the entire left atrium. This soft robotic sensor array (SRSA) system, once actuated (i.e., pressurized), forces the electrode array into contact with cardiac tissue without occluding blood flow. The soft actuators, made of compliant polyurethanes, will bend/stretch to adapt to each patient’s unique anatomy. The configurations are also well suited for adapting to complex atrial anatomies given their high level of compliance, and ability to undergo large deformations. The device leverages the advantages of soft robotic actuators and flexible electronic sensor arrays, both of which interface safely with tissue and conform to complex anatomy. The labs' central hypothesis: SRSAs will provide rapid and effective electrical mapping of electrograms from the entirety of patient atria.