Lab Focus, History, Achievements
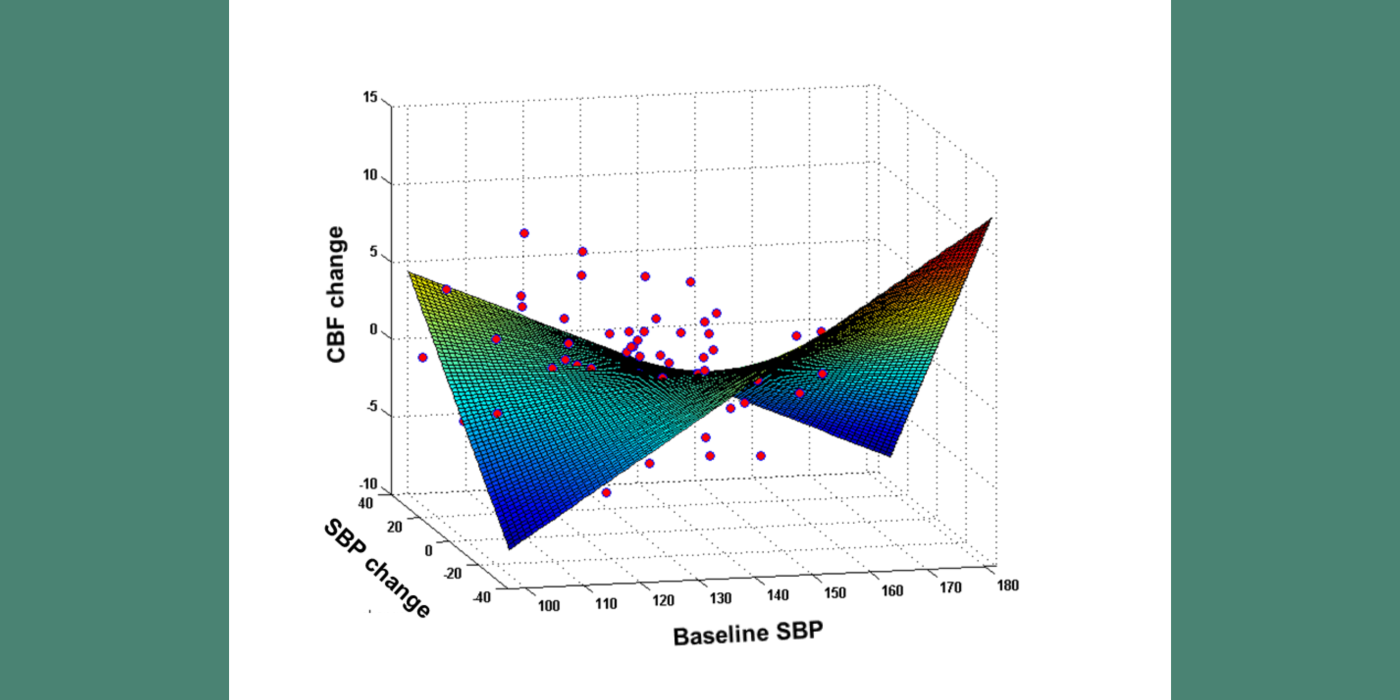
The VARIA (VAscular RIsk In Alzheimer's disease) Lab has described how hypertension (HTN) impairs cerebral blood flow (CBF) (which precedes cognitive decline), and the link between blood pressure and CBF. The lab has shown that, in subjects with hypertension, but not in normotensive individuals, there is an inverted U-shaped relationship between systolic blood pressure (SBP) and CBF, suggesting there is a blood pressure value in HTN that maximizes perfusion. This value was close to 125 mmHg for systolic blood pressure. Our longitudinal data confirmed this sweet spot, showing that moving away from the optimum SBP in both low and high BP directions was related to CBF reduction. Furthermore, we find that there is a blood pressure level where white matter lesion (WML) burden, a common manifestation of ischemic injury in hypertension and AD, is smallest. Most interestingly, the local maximum of the inverted U-shape curve for CBF, and the local minimum of the U-shaped curve for WML volume, are almost identical: around mid-120 systolic blood pressure. These findings support the existence of an ideal therapeutic target blood pressure maximizing brain function and minimizing damage.