Atrial Fibrillation and Unstable Cardiac Arrhythmia: Atrial fibrillation (AF) is a significant problem for the health care community. The most common cardiac arrhythmia, it impacts more than 35 million individuals worldwide. In this disorder, complex and aberrant electrical signals override normal cardiac electrical signals, causing the left atrium to contract rapidly and asynchronous to the cardiac cycle. This results in turbulent blood flow dramatically increasing stroke risk. For many patients, this can be managed through drug therapy; however, for many others, the only treatment is cardiac mapping.
Cardiac Mapping, Procedure and Limitations: In cardiac mapping, an electrophysiologist uses a minimally invasive diagnostic catheter with one or many sensors at the tip to collect electrical measurements from the heart’s interior surface. By moving the sensors around the surface, a comprehensive picture of the electrical activity is obtained. This way, the electrophysiologist identifies potential sources of the arrhythmia and uses a second catheter to deliver radiofrequency (RF) or cryothermal energy to eliminate arrhythmia sources. While cardiac mapping is a relatively effective procedure for many arrhythmias, its success with AF is limited, with 60% of patients experiencing symptom recurrence within a year. One important reason: the unstable nature of AF. Due to the complexity of the arrhythmia, signals collected at different time points cannot be easily combined. There is a need for devices that can accommodate large numbers of sensors to simultaneously collect signals from the entirety of the cardiac surface.
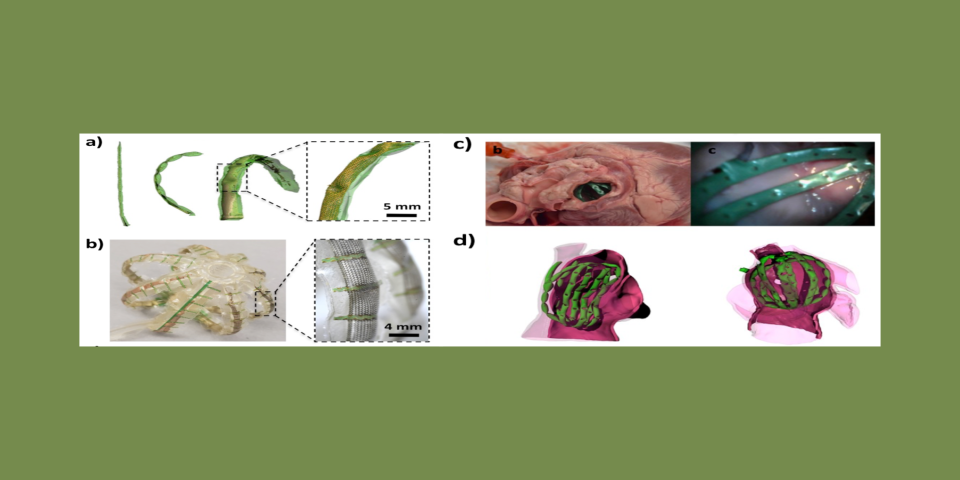
As minimally invasive catheter technology has advanced, there has been a push for technologies that integrate more sensors to provide more comprehensive pictures of arrhythmia. Devices on the market often incorporate large numbers of sensors (up to 128 electrodes), but their success is limited by their ability to conform to the complex anatomy of real patients’ hearts. Devices that aim to simultaneously map the entire cardiac surface typically deploy sensors in a spherical cage of inextensible metallic filaments. However, because the heart’s surface is complex, with pockets and crevasses throughout, when the devices are deployed, many sensors do not make effective contact with the surface and cannot acquire high-quality signals. Many sensors can also bunch in certain locations, leaving large areas unmapped. To address these limitations, our lab has developed a novel soft robotic sensor array that provides a comprehensive mapping of complex cardiac anatomies. Rather than relying on sensors deployed on conventional metallic filaments, these devices use soft actuators, fabricated from medical-grade polyurethanes. As these actuators are hydraulically driven they bend and stretch allowing them to conform to complex cardiac anatomies. Made from soft, flexible materials, they are intrinsically safe. By embedding arrays of flexible and stretchable sensors within the surface of these actuators, we create devices that effectively map electrical activity from complex cardiac surfaces. The image above shows several configurations of these devices in free space and deployed in cadaveric and 3D-printed cardiac environments.