Award or Grant: National Institutes of Health (NIH), R01 EB027918
The goal of this project is to develop noninvasive, safe, temporal monitoring of adeno-associated viral vector (AAV) biodistribution following in vivo administration that can be ultimately used in humans. The lab’s strategy is to covalently radioiodinate AAV capsids using the positron emitting isotope iodine (I)-124, and to track the labeled capsid using positron emission tomography (PET). The lab’s preliminary data in mice, rats and nonhuman primates have demonstrated the potential of assessing capsid biodistribution as a function of time for at least 10 days post-administration. For this project, the lab introduced the concept of in vivo viral vector dosimetry, defined in the context of 3D spatial-mapping of viral vector biodistribution at sub-centimeter spatial resolution anywhere in the body. The application of formal dosimetry and compartmental modeling principles yields the number of capsids delivered to a target organ from one or multiple routes and administrations. The degree to which vector biodistribution is a surrogate for both on- and off-target levels of transgene expression will be determined using biodistribution reporter gene standard assays, against which the lab will compare the noninvasive imaging.
For this project, the lab is evaluating multiple AAV serotypes, including four naturally occurring serotypes (AAV5, AAV8, AAV9 and AAVrh.10), commonly used in experimental animals and humans. As an example of a capsid-modified AAV serotype engineered to alter vector biodistribution, the lab will study AAV5-pK2, an AAV5 capsid genetically modified with lysine residues, markedly altering vector biodistribution when administered intravenously. In Aim 1, the lab will optimize its radiolabeling procedures and quality assurance procedures for the labeled vectors. In Aim 2, the lab will assess each of several serotype I-124 labeled vectors in nonhuman primates with no pre-existing immunity to the serotype of the test vector, using the common intravenous and intracisternal delivery routes. In Aim 3, the lab will investigate biodistribution in the context of immunity against each serotype.
A direct benefit of the project will be the ability to noninvasively monitor the effectiveness of AAV vector administration relevant to a wide range of AAV gene therapy trials. The lab’s methods should find special utility in rapidly assessing new capsid designs and different delivery routes for in vivo gene therapy. In addition, the lab’s methods will facilitate development of the new field of viral vector dosimetry, which will provide researchers and clinicians a quantitative tool for calculating vector delivery to organs in different gene therapy applications. Importantly, the methods the lab will develop are designed to facilitate noninvasive imaging well suited for translation to human use.
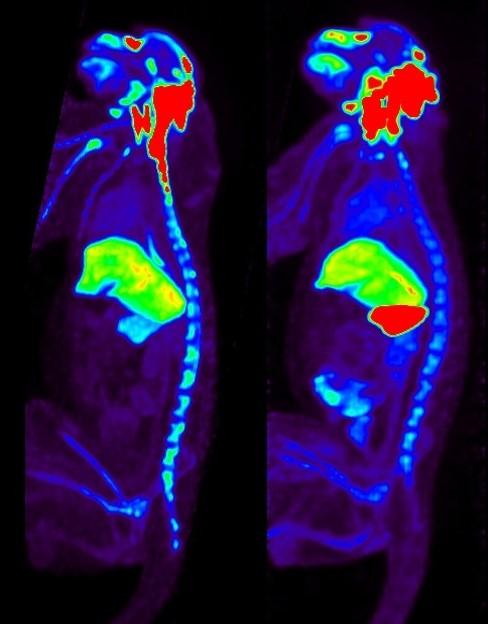
Full Caption: This image depicts the biodistribution of AAV. PET images 24 hours after I-124-labeled AAV serotype rh (rh)10 administered via intracisternal route to a nonhuman primate, showing differences in organ biodistribution between serotype naïve (L) and serotype immune (R) status. With anti-capsid immunity, uptake in spleen increased by more than a factor of 10.