Award or Grant: R21 NS116516; R01 NS123576-01A1 (not yet funded)
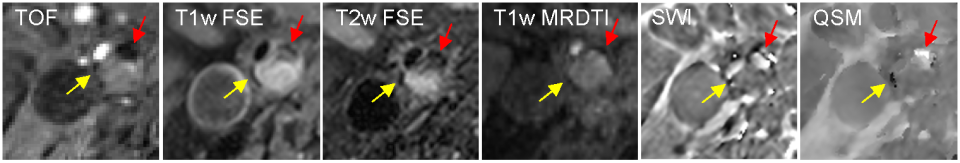
The team’s key objective is to use quantitative susceptibility mapping (QSM) to establish reliable, noninvasive magnetic resonance imaging (MRI) for identification and risk stratification of unstable carotid atherosclerotic plaques. Currently, decisions about carotid revascularization to prevent stroke, such as carotid endarterectomy or carotid artery stenting, are based on whether there is >50% carotid artery stenosis. However, this strategy uses only one feature of vulnerable plaque and frequently misclassifies patients. Using imaging to identify other features of rupture-prone carotid plaques with high risk for thromboembolic stroke, in combination with stenosis assessment, is a more effective approach for risk evaluation. Of these features, intraplaque hemorrhage (IPH) is associated with a four-to six- fold higher risk of stroke, while calcification is associated with a 50% lower stroke risk. In the conventional approach, IPH and calcification are defined as hyperintensity and hypointensity, respectively, in a plaque region on the T1-weighted (T1w) image acquired as part of the multi-contrast MRI (mcMRI) protocol.
However, T1w hyperintensity only captures the transient methemoglobin phase of hemorrhage. In the ensuing hemosiderin phase, IPH appears hypointense due to the strong susceptibility-induced dephasing effects of the superparamagnetic hemosiderin (susceptibility>150 ppm), which can be misinterpreted as calcification, although calcification is strongly diamagnetic (-2.3 ppm). The key scientific premise of this proposal is that QSM can reliably resolve T1w hypointensity into IPH hemosiderin versus calcification based on their different magnetic property, and therefore will significantly improve imaging characterization and risk stratification of patients with atherosclerotic carotid plaques. The lab has pioneered QSM development and demonstrated the exquisite sensitivity of QSM for hemorrhage and calcification in carotid plaque. The team will further improve the utility of carotid plaque QSM for routine clinical imaging by developing a multi-contrast QSM (mcQSM) approach which can provide not only QSM but also traditional mcMRI contrasts in five-minute scan time. The team will develop a nonlinear QSM reconstruction algorithm which is robust against noise and motion, and can separate co-existing IPH and calcification to improve IPH detection in calcified vessels. The team will then establish the improvement in diagnostic accuracy of mcQSM over mcMRI for detecting IPH and calcification in patients scheduled for carotid endarterectomy. Finally, the team will test the hypothesis that mcQSM will provide significantly higher discrimination for stroke than mcMRI. A successful outcome of this proposal will make carotid plaque QSM ready for widespread and routine clinical use in the emerging era of personalized medicine to reduce the individual and societal burden of stroke.