Award or Grant: R01 AG077594-01 (not yet funded)
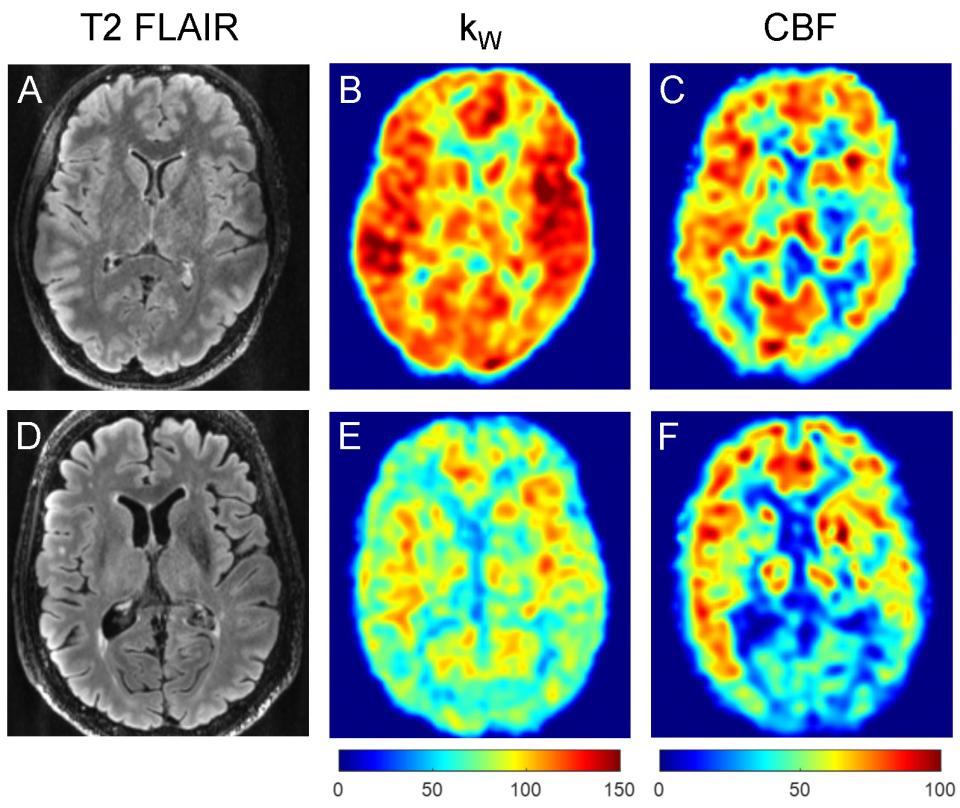
The team’s objective is to develop quantitative permeability mapping (QPM) of kW, the water exchange rate across the blood-brain barrier (BBB), for the detection and quantification of BBB impairment in normal aging and Alzheimer’s disease (AD). Dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) can be used to map BBB permeability to an exogenous gadolinium-based contrast agent (GBCA), expressed as kTRANS, the increase of which has been shown to precede amyloid beta (Aβ) and tau deposition and structural atrophy in normal aging and AD. However, kTRANS only measures GBCA leakage across the endothelial tight junctions and does not capture the transport of much smaller water molecules through water-selective channels mediated by aquaporin-4 (AQP4) on astrocyte end-feet. AQP4 expression is known to decrease with age and associated with cognitive decline, but it has been challenging to quantify AQP4-mediated water transport in vivo.
The team recently demonstrated the feasibility of a high-resolution QPM pulse sequence with robust adiabatic diffusion preparation and efficient spiral fast spin-echo readout for whole-brain kW mapping. The team has shown that kW, which can only be mapped by using water as an endogenous tracer, also decreases with age and is potentially an imaging biomarker of impaired water transport across the BBB. The team’s approach is to develop QPM for robust kW mapping, establish the biological correlates of kW, and evaluate the diagnostic and prognostic utility of kW in AD patients and cognitive normal (CN) controls. In the proposed research, the team will improve the robustness of QPM-derived kW mapping, correlate kW with age, sex, and APO-E4 genotype, and with cerebrospinal fluid (CSF) biomarkers and Aβ PET in CN subjects, and evaluate the diagnostic and prognostic utility of kW in AD patients. A successful outcome of this project will improve understanding of BBB impairment, laying the foundation for the development of an early disease biomarker, illuminating potential therapeutic targets, and improving subject selection in AD therapeutic clinical trials.