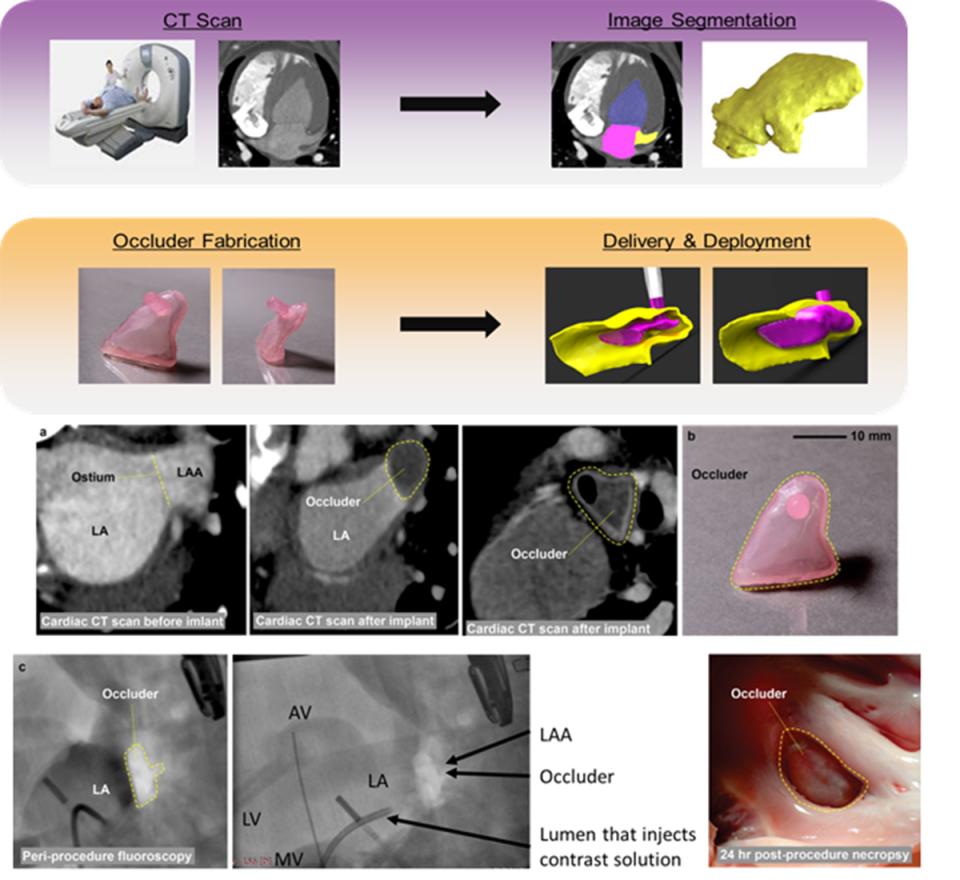
The goal of this project was to develop a patient-specific occluder for the left atrial appendage (LAA), as a stroke prevention device for atrial fibrillation (AF) patients. The lab developed a workflow to design and fabricate personalized devices using 3D printing. AF, which promotes blood clots, increases stroke risk five to seven times over. Warfarin, an oral anti-coagulant, is the mainstay of treatment for reducing stroke risk due to AF. This therapy, however, is systemic and increases the risk of bleeding dramatically. So alternative treatments that locally prevent thrombi development in the atrium are desirable. The LAA is the source of more than 90% of thrombi in patients with non-rheumatic AF. Surgical closure of the LAA offers a non-pharmacologic alternative to anticoagulant medications for stroke prevention in AF patients, but it is highly invasive and only effective in ~40% of cases.
Percutaneous transcatheter techniques have been developed (e.g., Watchman, Lariat, Amulet) as less invasive approaches to LAA closure. These devices cut stroke risk, but they are often associated with other risks, limiting adoption. These include: blood leakage due to inadequate LAA sealing; device dislodgement; pericardial effusion; and thrombosis. There are two key reasons for the limitations. One: the size/shape of the LAA varies dramatically among patients, making it difficult for standard devices to fully seal the appendage. The overall volume of the LAA varies between 0.77 and 19.27 cm3. The shape also varies, resulting in the broad classification of six types. These anatomic variations have led to patient exclusions from treatment. A study evaluating the Watchman device found ~23% of patients should be excluded based on anatomy. The second reason for limitations: the use of metal (nitinol) in the devices, which can serve as a nidus for thrombus formation and cause LAA puncture.
The Mosadegh group published, in Nature Biomedical Engineering ((January 2018) a method to address the above issues by creating a patient-specific LAA occluder fabricated by casting elastomeric materials in 3D-printed molds, creating a soft inflatable occluder. The occluder’s patient-specificity was achieved by (i) the geometry of the occluder matching the morphology of the LAA based on a computed tomography scan, and (ii) the use of ultra-compliant materials (extensibility >400%) conforming to the irregular shape of the LAA upon inflation. The method’s feasibility was shown by surgically implanting the patient-specific occluder in a large animal model.