Clinical trial-based research to expand the use of alpha particle-based therapeutics. Actinium-225 is a radiopharmaceutical we will be producing in the first-of-its-kind alpha labeling lab in an academic institution. The image demonstrates an example of how we hope to benefit patients seeking cancer care through this institutional lab.
IRB Approvals:
IRB #AML02. A Phase I/II Study of Venetoclax and Lintuzumab-Ac225 in Patients with Refractory or Relapsed AML
IRB #21-01023145. SPLASH: Study Evaluating Metastatic Castrate Resistant Prostate Cancer Treatment Using 177Lu-PNT2002 PSMA Therapy after Second-line Hormonal Treatment
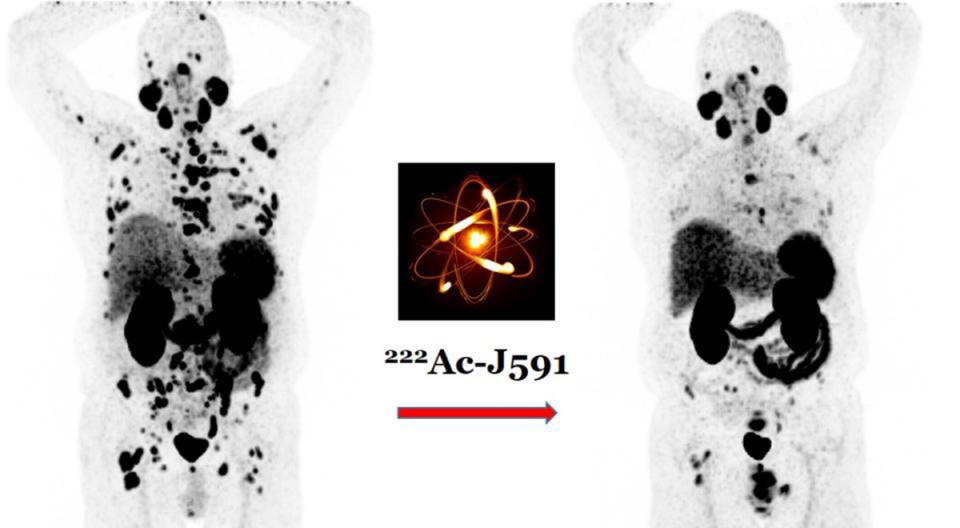
IRB # 20-01021288. Phase I/II dose-escalation study of fractionated and multiple-dose 225Ac-J591 for progressive metastatic castration-resistant prostate cancer
Publications and Presentations:
Tagawa, S., et al., Phase I dose-escalation study of PSMA-targeted alpha emitter <sup>225</sup>Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Onc., 38(15_suppl), pp.5560-5560: 2020.
Tagawa S.T., et al., 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177 Lu-J591) for metastatic castration-resistant prostate cancer, Cancer: 2019 Aug 1;125(15):2561-2569.
Tagawa, S., et al.. Dose-escalation results of a phase I study of 225Ac-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Onc., 38(6_suppl), pp.114-114: 2020.
Tagawa, S., et al., 952 Anti-prostate specific membrane antigen (PSMA)-based radioimmunotherapy for metastatic castration-resistant prostate cancer (CRPC): A decade of experience with radiolabeled (RL)-J591. J. Urol., 187(4S): 2012.