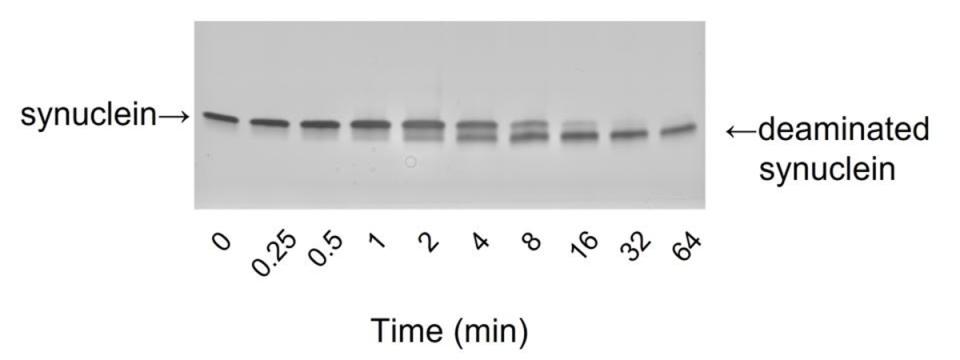
In addition to crosslinking, transglutaminases catalyze deamination, or the removal of amine groups from proteins. This reaction accounts for celiac disease. In this disease, intestinal transglutaminases deaminate gliadin. The deaminated gliadin is then recognized as foreign by the immune system, and the ensuing inflammatory response causes celiac disease. The Jeitner lab discovered that α-synuclein, the protein aggregated in Parkinson’s disease, is also deaminated by tissue transgluaminase (TG2). As α-synuclein is an unordered protein, the lab hypothesizes that deaminated α-synuclein may have sufficient order to self-assemble in the gut, thereby, according to the Braak Hypothesis, initiating Parkinson’s disease.