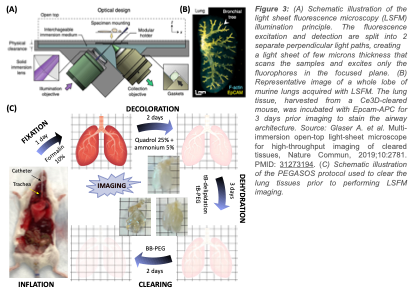
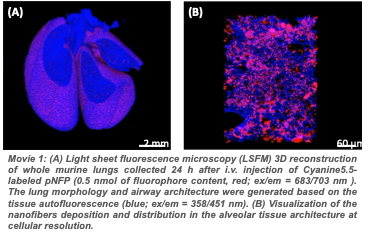
The main limitations of the use of nanoparticles as drug delivery platform for cancer treatment are the inconsistent deep-tissue targeting resulting in a lack of tumor penetration, and the incomplete drug release on tumor site leading to a depreciation of safety and therapeutic efficacy. The nanofiber technology allows to carry more drugs to the metastatic lung tumors and favors the local accumulation and retention of antitumor medication. However, an effective drug carrier must also release the active pharmaceutical at the target-site to maximize the therapeutic index. To investigate how the nanofiber’s post-delivery distribution and drug release profile affect the treatment outcome, we will notably use Light Sheet Fluorescence Microscopy (Figure A). LSFM is an emerging optical imaging technique suitable for visualizing and measuring a dynamic biological process in vivo. This microscope allows the mapping of whole murine organs (Figure B) and has been used to evaluate the distribution of nanoparticles within multiple tissues and monitor the penetration of small drug molecules, into multicellular tumor spheroids. After optical clearing of the diseased lungs using the PEGASOS protocol (Figure C), this imaging technique will allow to image concurrently the 3D metastasis architecture, the pNFP biodistribution within the pulmonary tissue (movies 1 and 2), and the drug release over time. This project will provide relevant new insights for a more accurate understanding of the complexity of the in vivo drug release mechanisms and kinetics. It will help us to optimize the design of the nanofibers to successfully release the drug payload in response to metastatic lung tumors. The information collected will be also instrumental for delivering other chemotherapeutics or drug molecules to the lungs.
More Information:

